Sodium-calcium exchange does not require allosteric calcium activation at high cytosolic sodium concentrations
- PMID: 16809364
- PMCID: PMC1995697
- DOI: 10.1113/jphysiol.2006.113910
Sodium-calcium exchange does not require allosteric calcium activation at high cytosolic sodium concentrations
Abstract
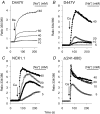
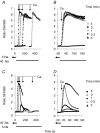
The activity of the cardiac Na(+)-Ca(2+) exchanger (NCX1.1) is allosterically regulated by Ca(2+), which binds to two acidic regions in the cytosolically disposed central hydrophilic domain of the NCX protein. A mutation in one of the regulatory Ca(2+) binding regions (D447V) increases the half-activation constant (K(h)) for allosteric Ca(2+) activation from approximately 0.3 to > 1.8 microm. Chinese hamster ovary cells expressing the D447V exchanger showed little or no activity under physiological ionic conditions unless cytosolic [Ca(2+)] was elevated to > 1 microm. However, when cytosolic [Na(+)] was increased to 20 mm or more (using ouabain-induced inhibition of the Na(+),K(+)-ATPase or the ionophore gramicidin), cells expressing the D447V mutant rapidly accumulated Ca(2+) or Ba(2+) when the reverse (Ca(2+) influx) mode of NCX activity was initiated, although initial cytosolic [Ca(2+)] was < 100 nm. Importantly, the time course of Ca(2+) uptake did not display the lag phase that reflects allosteric Ca(2+) activation of NCX activity in the wild-type NCX1.1; indeed, at elevated [Na(+)], the D447V mutant behaved similarly to the constitutively active deletion mutant Delta(241-680), which lacks the regulatory Ca(2+) binding sites. In cells expressing wild-type NCX1.1, increasing concentrations of cytosolic Na(+) led to a progressive shortening of the lag phase for Ca(2+) uptake. The effects of elevated [Na(+)] developed rapidly and were fully reversible. The activity of the D447V mutant was markedly inhibited when phosphatidylinositol 4,5-bisphosphate (PIP2) levels were reduced. We conclude that when PIP2 levels are high, elevated cytosolic [Na(+)] induces a mode of exchange activity that does not require allosteric Ca(2+) activation.
Figures





References
-
- Balla T. Phosphoinositide-derived messengers in endocrine signaling. J Endocrinol. 2006;188:135–153. - PubMed
-
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. - PubMed
-
- Chernysh O, Condrescu M, Reeves JP. Calcium-dependent regulation of calcium efflux by the cardiac sodium/calcium exchanger. Am J Physiol Cell Physiol. 2004;287:C797–C806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

