Recurrent inhibition of the bladder C fibre reflex in the cat and its response to naloxone
- PMID: 16809367
- PMCID: PMC1819444
- DOI: 10.1113/jphysiol.2006.112995
Recurrent inhibition of the bladder C fibre reflex in the cat and its response to naloxone
Abstract
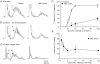
Recurrent inhibition of the bladder C fibre reflex was studied in adult female cats anaesthetized with alpha-chloralose. Test reflexes were evoked by electrical stimulation of bladder Adelta and C afferents in the right pelvic nerve and were recorded from the proximal end of a small ipsilateral pelvic nerve branch, transected close to the bladder. Such test reflexes were consistently depressed by repetitive electrical stimulation of the contralateral bladder pelvic nerve (20 Hz, 20 s) at intensities sufficient to recruit axons of bladder preganglionic neurones. The inhibition could be evoked after transection of the left dorsal roots S1-S4 and the sympathetic supply to the bladder but was abolished by transection of the pelvic nerve central to the site of stimulation. Hence, it most likely involved central recurrent collaterals of antidromically activated bladder preganglionic neurones. The reflex suppression was quite considerable - maximal C fibre reflexes were reduced to a group mean of 25% (+/- 9% confidence interval) of their control size. The effect had a slow onset, requiring a few seconds of conditioning stimulation to be revealed, and was very long lasting (minutes). Naloxone (0.01-0.5 mg kg(-1) i.v.) abolished the recurrent inhibition of both the C fibre and Adelta bladder reflexes, while inhibition from afferents in the dorsal clitoris nerve remained unchanged. It is concluded that the segmental bladder C fibre reflex and the spino-ponto-spinal Adelta micturition reflex are both targets of recurrent inhibition from bladder parasympathetic preganglionic neurones and that the effect involves an enkephalinergic mechanism.
Figures



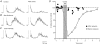
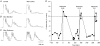

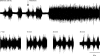

References
-
- Blok BF, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to the parasympathetic preganglionic motoneurons of the bladder of the cat. Neuroscience Lett. 1997;222:195–198. - PubMed
-
- Cruz F. Mechanisms involved in new therapies for overactive bladder. Urology. 2004;63:65–73. - PubMed
-
- De Groat WC, Booth AM, Milne RJ, Roppolo JR. Parasympathetic preganglionic neurons in the sacral spinal cord. J Autonomic Nervous System. 1982;5:23–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

