Long term effects of azithromycin in patients with cystic fibrosis: A double blind, placebo controlled trial
- PMID: 16809416
- PMCID: PMC2104771
- DOI: 10.1136/thx.2005.057950
Long term effects of azithromycin in patients with cystic fibrosis: A double blind, placebo controlled trial
Abstract
Background: Macrolides display immunomodulatory effects that may be beneficial in chronic inflammatory pulmonary diseases. The aim of the study was to document whether long term use of azithromycin may be associated with respiratory benefits in young patients with cystic fibrosis.
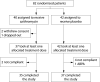
Methods: A multicentre, randomised, double blind, placebo controlled trial was conducted from October 2001 to June 2003. The criteria for enrollment were age older than 6 years and forced expiratory volume in 1 second (FEV1) of 40% or more. The active group received either 250 mg or 500 mg (body weight < or > or =40 kg) of oral azithromycin three times a week for 12 months. The primary end point was change in FEV1.
Results: Eighty two patients of mean (SD) age 11.0 (3.3) years and mean (SD) FEV1 85 (22)% predicted were randomised: 40 in the azithromycin group and 42 in the placebo group. Nineteen patients were infected with Pseudomonas aeruginosa. The relative change in FEV1 at month 12 did not differ significantly between the two groups. The number of pulmonary exacerbations (count ratio 0.50 (95% CI 0.32 to 0.79), p < 0.005), the time elapsed before the first pulmonary exacerbation (hazard ratio 0.37 (95% CI 0.22 to 0.63), p < 0.0001), and the number of additional courses of oral antibiotics were significantly reduced in the azithromycin group regardless of the infectious status (count ratio 0.55 (95% CI 0.36 to 0.85), p < 0.01). No severe adverse events were reported.
Conclusion: Long term use of low dose azithromycin in young patients with cystic fibrosis has a beneficial effect on lung disease expression, even before infection with Pseudomonas aeruginosa.
Conflict of interest statement
Competing interests: none declared.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical