Lhx2 maintains stem cell character in hair follicles
- PMID: 16809539
- PMCID: PMC2405918
- DOI: 10.1126/science.1128004
Lhx2 maintains stem cell character in hair follicles
Abstract
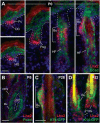
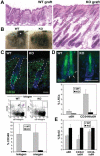
During embryogenesis, stem cells are set aside to fuel the postnatal hair cycle and repair the epidermis after injury. To define how hair follicle stem cells are specified and maintained in an undifferentiated state, we developed a strategy to isolate and transcriptionally profile embryonic hair progenitors in mice. We identified Lhx2 as a transcription factor positioned downstream of signals necessary to specify hair follicle stem cells, but upstream from signals required to drive activated stem cells to terminally differentiate. Using gain- and loss-of-function studies, we uncovered a role for Lhx2 in maintaining the growth and undifferentiated properties of hair follicle progenitors.
Figures



References
-
- Hardy MH. Trends Genet. 1992;8:55. - PubMed
-
- Millar SE. J. Invest. Dermatol. 2002;118:216. - PubMed
-
- Schmidt-Ullrich R, Paus R. Bioessays. 2005;27:247. - PubMed
-
- DasGupta R, Fuchs E. Development. 1999;126:4557. - PubMed
-
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Cell. 2001;105:533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

