BCL11B participates in the activation of IL2 gene expression in CD4+ T lymphocytes
- PMID: 16809611
- PMCID: PMC1895584
- DOI: 10.1182/blood-2006-05-021790
BCL11B participates in the activation of IL2 gene expression in CD4+ T lymphocytes
Abstract
BCL11A and BCL11B are transcriptional regulators important for lymphopoiesis and previously associated with hematopoietic malignancies. Ablation of the mouse Bcl11b locus results in failure to generate double-positive thymocytes, implicating a critical role of Bcl11b in T-cell development. However, BCL11B is also expressed in CD4+ T lymphocytes, both in resting and activated states. Here we show both in transformed and primary CD4+ T cells that BCL11B participates in the control of the interleukin-2 (IL2) gene expression following activation through T-cell receptor (TCR). BCL11B augments expression from the IL2 promoter through direct binding to the US1 site. In addition, BCL11B associates with the p300 coactivator in CD4+ T cells activated through TCR, which may account for its transcriptional activation function. These results provide the first evidence that BCL11B, originally described as a transcriptional repressor, activates transcription of a target gene in the context of T-cell activation.
Figures
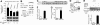


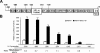

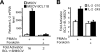
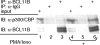
Similar articles
-
BCL11B enhances TCR/CD28-triggered NF-kappaB activation through up-regulation of Cot kinase gene expression in T-lymphocytes.Biochem J. 2009 Jan 15;417(2):457-66. doi: 10.1042/BJ20080925. Biochem J. 2009. PMID: 18831712 Free PMC article.
-
Protein Kinase C-Mediated Phosphorylation of BCL11B at Serine 2 Negatively Regulates Its Interaction with NuRD Complexes during CD4+ T-Cell Activation.Mol Cell Biol. 2016 Jun 15;36(13):1881-98. doi: 10.1128/MCB.00062-16. Print 2016 Jul 1. Mol Cell Biol. 2016. PMID: 27161321 Free PMC article.
-
BCL11B functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter.Oncogene. 2005 Oct 13;24(45):6753-64. doi: 10.1038/sj.onc.1208904. Oncogene. 2005. PMID: 16091750
-
Role of the transcription factor Bcl11b in development and lymphomagenesis.Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(3):72-87. doi: 10.2183/pjab.88.72. Proc Jpn Acad Ser B Phys Biol Sci. 2012. PMID: 22450536 Free PMC article. Review.
-
Epigenetic Dynamics in the Function of T-Lineage Regulatory Factor Bcl11b.Front Immunol. 2021 Apr 14;12:669498. doi: 10.3389/fimmu.2021.669498. eCollection 2021. Front Immunol. 2021. PMID: 33936112 Free PMC article. Review.
Cited by
-
Retroviral insertions in the VISION database identify molecular pathways in mouse lymphoid leukemia and lymphoma.Mamm Genome. 2007 Oct;18(10):709-22. doi: 10.1007/s00335-007-9060-2. Epub 2007 Oct 10. Mamm Genome. 2007. PMID: 17926094 Free PMC article.
-
Transcription factor Bcl11b controls selection of invariant natural killer T-cells by regulating glycolipid presentation in double-positive thymocytes.Proc Natl Acad Sci U S A. 2011 Apr 12;108(15):6211-6. doi: 10.1073/pnas.1014304108. Epub 2011 Mar 28. Proc Natl Acad Sci U S A. 2011. PMID: 21444811 Free PMC article.
-
The DN2 Myeloid-T (DN2mt) Progenitor is a Target Cell for Leukemic Transformation by the TLX1 Oncogene.J Bone Marrow Res. 2013 Feb 20;1:105. doi: 10.4172/2329-8820.1000105. J Bone Marrow Res. 2013. PMID: 25309961 Free PMC article.
-
Intrinsic Disorder of the BAF Complex: Roles in Chromatin Remodeling and Disease Development.Int J Mol Sci. 2019 Oct 23;20(21):5260. doi: 10.3390/ijms20215260. Int J Mol Sci. 2019. PMID: 31652801 Free PMC article.
-
The multifaceted roles of Bcl11b in thymic and peripheral T cells: impact on immune diseases.J Immunol. 2014 Sep 1;193(5):2059-65. doi: 10.4049/jimmunol.1400930. J Immunol. 2014. PMID: 25128552 Free PMC article. Review.
References
-
- Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem. 2000;275: 10315-10322. - PMC - PubMed
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45: 207-221. - PubMed
-
- Wakabayashi Y, Watanabe H, Inoue J, et al. Bcl11b is required for differentiation and survival of αβ T lymphocytes. Nat Immunol. 2003;4: 533-539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

