Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery
- PMID: 16809617
- PMCID: PMC1895582
- DOI: 10.1182/blood-2006-03-012534
Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery
Erratum in
- Blood. 2007 Mar 1;109(5):1816
Abstract
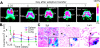
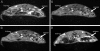
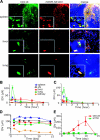
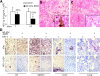
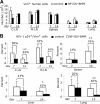
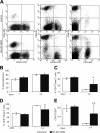
Complex dosing regimens, costs, side effects, biodistribution limitations, and variable drug pharmacokinetic patterns have affected the long-term efficacy of antiretroviral medicines. To address these problems, a nanoparticle indinavir (NP-IDV) formulation packaged into carrier bone marrow-derived macrophages (BMMs) was developed. Drug distribution and disease outcomes were assessed in immune-competent and human immunodeficiency virus type 1 (HIV-1)-infected humanized immune-deficient mice, respectively. In the former, NP-IDV formulation contained within BMMs was adoptively transferred. After a single administration, single-photon emission computed tomography, histology, and reverse-phase-high-performance liquid chromatography (RP-HPLC) demonstrated robust lung, liver, and spleen BMMs and drug distribution. Tissue and sera IDV levels were greater than or equal to 50 microM for 2 weeks. NP-IDV-BMMs administered to HIV-1-challenged humanized mice revealed reduced numbers of virus-infected cells in plasma, lymph nodes, spleen, liver, and lung, as well as, CD4(+) T-cell protection. We conclude that a single dose of NP-IDV, using BMMs as a carrier, is effective and warrants consideration for human testing.
Figures

Similar articles
-
Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS.J Immunol. 2009 Jul 1;183(1):661-9. doi: 10.4049/jimmunol.0900274. Epub 2009 Jun 17. J Immunol. 2009. PMID: 19535632 Free PMC article.
-
Maintenance of indinavir by dose adjustment in HIV-1-infected patients with indinavir-related toxicity.Eur J Clin Pharmacol. 2007 Oct;63(10):901-8. doi: 10.1007/s00228-007-0343-z. Epub 2007 Aug 10. Eur J Clin Pharmacol. 2007. PMID: 17690876 Clinical Trial.
-
Therapeutic drug monitoring of indinavir in HIV-infected patients undergoing HAART.Infection. 2002 Jan;30(1):13-6. doi: 10.1007/s15010-001-1111-0. Infection. 2002. PMID: 11876509
-
Pharmacokinetics of indinavir and ritonavir administered at 667 and 100 milligrams, respectively, every 12 hours compared with indinavir administered at 800 milligrams every 8 hours in human immunodeficiency virus-infected patients.Antimicrob Agents Chemother. 2004 Nov;48(11):4200-8. doi: 10.1128/AAC.48.11.4200-4208.2004. Antimicrob Agents Chemother. 2004. PMID: 15504842 Free PMC article. Clinical Trial.
-
Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients.AIDS. 2000 Dec 22;14(18):2869-76. doi: 10.1097/00002030-200012220-00008. AIDS. 2000. PMID: 11153668 Clinical Trial.
Cited by
-
Nanomedicine in Central Nervous System (CNS) Disorders: A Present and Future Prospective.Adv Pharm Bull. 2016 Sep;6(3):319-335. doi: 10.15171/apb.2016.044. Epub 2016 Sep 25. Adv Pharm Bull. 2016. PMID: 27766216 Free PMC article. Review.
-
Treatment of Neonatal Hypoxic-Ischemic Encephalopathy with Erythropoietin Alone, and Erythropoietin Combined with Hypothermia: History, Current Status, and Future Research.Int J Mol Sci. 2020 Feb 21;21(4):1487. doi: 10.3390/ijms21041487. Int J Mol Sci. 2020. PMID: 32098276 Free PMC article. Review.
-
Harnessing Macrophages for Controlled-Release Drug Delivery: Lessons From Microbes.Front Pharmacol. 2019 Jan 25;10:22. doi: 10.3389/fphar.2019.00022. eCollection 2019. Front Pharmacol. 2019. PMID: 30740053 Free PMC article. Review.
-
Cell delivery of therapeutic nanoparticles.Prog Mol Biol Transl Sci. 2011;104:563-601. doi: 10.1016/B978-0-12-416020-0.00014-0. Prog Mol Biol Transl Sci. 2011. PMID: 22093229 Free PMC article.
-
Long-acting slow effective release antiretroviral therapy.Expert Opin Drug Deliv. 2017 Nov;14(11):1281-1291. doi: 10.1080/17425247.2017.1288212. Epub 2017 Feb 6. Expert Opin Drug Deliv. 2017. PMID: 28128004 Free PMC article. Review.
References
-
- Dam Nielsen S, Kjaer Ersboll A, Mathiesen L, Nielsen JO, Hansen JE. Highly active antiretroviral therapy normalizes the function of progenitor cells in human immunodeficiency virus-infected patients. J Infect Dis. 1998;178: 1299-1305. - PubMed
-
- Pezzotti P, Napoli PA, Acciai S, et al. Increasing survival time after AIDS in Italy: the role of new combination antiretroviral therapies: Tuscany AIDS Study Group. Aids. 1999;13: 249-255. - PubMed
-
- Sharland M, Watkins AM, Dalgleish AG, Cammack N, Westby M. Immune reconstitution in HAART-treated children with AIDS: Highly Active Anti-Retroviral Therapy. Lancet. 1998;352: 577-578. - PubMed
-
- Piot P, Bartos M, Ghys PD, Walker N, Schwartlander B. The global impact of HIV/AIDS. Nature. 2001;410: 968-973. - PubMed
-
- Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutrition. 2005;21: 96-99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2R37 NS 36126/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P20 RR 15635/RR/NCRR NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 MH 64570/MH/NIMH NIH HHS/United States
- 2R01 NS 034239/NS/NINDS NIH HHS/United States
- P01 NS 31492/NS/NINDS NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P01 NS 43985/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

