Mechanistic studies of the mitotic activation of Mos
- PMID: 16809767
- PMCID: PMC1592720
- DOI: 10.1128/MCB.00273-06
Mechanistic studies of the mitotic activation of Mos
Abstract
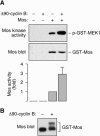
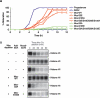
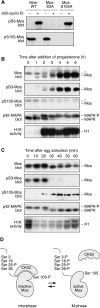
The protein kinase Mos is responsible for the activation of MEK1 and p42 mitogen-activated protein kinase during Xenopus oocyte maturation and during mitosis in Xenopus egg extracts. Here we show that the activation of Mos depends upon the phosphorylation of Ser 3, a residue previously implicated in the regulation of Mos stability; the dephosphorylation of Ser 105, a previously unidentified phosphorylation site conserved in Mos proteins; and the regulated dissociation of Mos from CK2beta. Mutation of Ser 3 to alanine and/or mutation of Ser 105 to glutamate produces a Mos protein that is defective for M-phase activation, as assessed by in vitro kinase assays, and defective for induction of oocyte maturation and maintenance of the spindle assembly checkpoint in extracts. Interestingly, Ser 105 is situated at the beginning of helix alphaC in the N-terminal lobe of the Mos kinase domain. Changes in the orientation of this helix have been previously implicated in the activation of Cdk2 and Src family tyrosine kinases. Our work suggests that Ser 105 dephosphorylation represents a novel mechanism for reorienting helix alphaC.
Figures



Similar articles
-
Mos mediates the mitotic activation of p42 MAPK in Xenopus egg extracts.Curr Biol. 2004 Sep 7;14(17):1581-6. doi: 10.1016/j.cub.2004.08.056. Curr Biol. 2004. PMID: 15341746
-
Mos-induced p42 mitogen-activated protein kinase activation stabilizes M-phase in Xenopus egg extracts after cyclin destruction.Biol Cell. 1998 Nov;90(8):565-72. Biol Cell. 1998. PMID: 10069001
-
A p90(rsk) mutant constitutively interacting with MAP kinase uncouples MAP kinase from p34(cdc2)/cyclin B activation in Xenopus oocytes.Mol Biol Cell. 1999 Sep;10(9):2971-86. doi: 10.1091/mbc.10.9.2971. Mol Biol Cell. 1999. PMID: 10473640 Free PMC article.
-
Across the meiotic divide - CSF activity in the post-Emi2/XErp1 era.J Cell Sci. 2008 Nov 1;121(Pt 21):3509-14. doi: 10.1242/jcs.036855. J Cell Sci. 2008. PMID: 18946022 Review.
-
The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro.Methods Cell Biol. 1999;61:385-412. doi: 10.1016/s0091-679x(08)61991-3. Methods Cell Biol. 1999. PMID: 9891325 Review. No abstract available.
Cited by
-
Search for the genes involved in oocyte maturation and early embryo development in the hen.BMC Genomics. 2008 Feb 29;9:110. doi: 10.1186/1471-2164-9-110. BMC Genomics. 2008. PMID: 18312645 Free PMC article.
-
A novel fluorescent cell membrane-permeable caged cyclic ADP-ribose analogue.J Biol Chem. 2012 Jul 13;287(29):24774-83. doi: 10.1074/jbc.M111.329854. Epub 2012 Jun 1. J Biol Chem. 2012. PMID: 22661714 Free PMC article.
-
Two pore channel 2 (TPC2) inhibits autophagosomal-lysosomal fusion by alkalinizing lysosomal pH.J Biol Chem. 2013 Aug 16;288(33):24247-63. doi: 10.1074/jbc.M113.484253. Epub 2013 Jul 8. J Biol Chem. 2013. Retraction in: J Biol Chem. 2017 Jul 21;292(29):12088. doi: 10.1074/jbc.A113.484253. PMID: 23836916 Free PMC article. Retracted.
-
Kicked by Mos and tuned by MPF-the initiation of the MAPK cascade in Xenopus oocytes.HFSP J. 2009 Dec;3(6):428-40. doi: 10.2976/1.3265771. Epub 2009 Dec 18. HFSP J. 2009. PMID: 20514133 Free PMC article.
-
Two pore channel 2 differentially modulates neural differentiation of mouse embryonic stem cells.PLoS One. 2013 Jun 12;8(6):e66077. doi: 10.1371/journal.pone.0066077. Print 2013. PLoS One. 2013. PMID: 23776607 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
