Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction
- PMID: 16809768
- PMCID: PMC1592718
- DOI: 10.1128/MCB.00084-06
Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction
Abstract
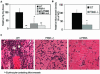
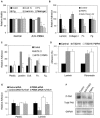
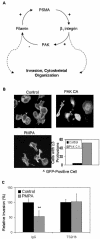
The transmembrane peptidase prostate-specific membrane antigen (PSMA) is universally upregulated in the vasculature of solid tumors, but its functional role in tumor angiogenesis has not been investigated. Here we show that angiogenesis is severely impaired in PSMA-null animals and that this angiogenic defect occurs at the level of endothelial cell invasion through the extracellular matrix barrier. Because proteolytic degradation of the extracellular matrix is a critical component of endothelial invasion in angiogenesis, it is logical to assume that PSMA participates in matrix degradation. However, we demonstrate a novel and more complex role for PSMA in angiogenesis, where it is a principal component of a regulatory loop that is tightly modulating laminin-specific integrin signaling and GTPase-dependent, p21-activated kinase 1 (PAK-1) activity. We show that PSMA inhibition, knockdown, or deficiency decreases endothelial cell invasion in vitro via integrin and PAK, thus abrogating angiogenesis. Interestingly, the neutralization of beta(1) or the inactivation of PAK increases PSMA activity, suggesting that they negatively regulate PSMA. This negative regulation is mediated by the cytoskeleton as the disruption of interactions between the PSMA cytoplasmic tail and the anchor protein filamin A decreases PSMA activity, integrin function, and PAK activation. Finally, the inhibition of PAK activation enhances the PSMA/filamin A interaction and, thus, boosts PSMA activity. These data imply that PSMA participates in an autoregulatory loop, wherein active PSMA facilitates integrin signaling and PAK activation, leading to both productive invasion and downregulation of integrin beta(1) signaling via reduced PSMA activity. Therefore, we have identified a novel role for PSMA as a true molecular interface, integrating both extracellular and intracellular signals during angiogenesis.
Figures





References
-
- Anilkumar, G., S. A. Rajasekaran, S. Wang, O. Hankinson, N. H. Bander, and A. K. Rajasekaran. 2003. Prostate-specific membrane antigen association with filamin A modulates its internalization and NAALADase activity. Cancer Res. 63:2645-2648. - PubMed
-
- Arroyo, A. G., A. Garcia-Pardo, and F. Sanchez-Madrid. 1993. A high affinity conformational state on VLA integrin heterodimers induced by an anti-β1 chain monoclonal antibody. J. Biol. Chem. 268:9863-9868. - PubMed
-
- Bacich, D. J., E. Ramadan, D. S. O'Keefe, N. Bukhari, I. Wegorzewska, O. Ojeifo, R. Olszewski, C. C. Wrenn, T. Bzdega, B. Wroblewska, W. D. Heston, and J. H. Neale. 2002. Deletion of the glutamate carboxypeptidase II gene in mice reveals a second enzyme activity that hydrolyzes N-acetylaspartylglutamate. J. Neurochem. 83:20-29. - PubMed
-
- Barker, J. N. 1991. The pathophysiology of psoriasis. Lancet 338:227-230. - PubMed
-
- Bauvois, B. 2004. Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis? Oncogene 23:317-329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
