Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors
- PMID: 16809784
- PMCID: PMC1592704
- DOI: 10.1128/MCB.00625-06
Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors
Abstract
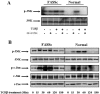
The signal transduction mechanisms generating pathological fibrosis are almost wholly unknown. Endothelin-1 (ET-1), which is up-regulated during tissue repair and fibrosis, induces lung fibroblasts to produce and contract extracellular matrix. Lung fibroblasts isolated from scleroderma patients with chronic pulmonary fibrosis produce elevated levels of ET-1, which contribute to the persistent fibrotic phenotype of these cells. Transforming growth factor beta (TGF-beta) induces fibroblasts to produce and contract matrix. In this report, we show that TGF-beta induces ET-1 in normal and fibrotic lung fibroblasts in a Smad-independent ALK5/c-Jun N-terminal kinase (JNK)/Ap-1-dependent fashion. ET-1 induces JNK through TAK1. Fibrotic lung fibroblasts display constitutive JNK activation, which was reduced by the dual ETA/ETB receptor inhibitor, bosentan, providing evidence of an autocrine endothelin loop. Thus, ET-1 and TGF-beta are likely to cooperate in the pathogenesis of pulmonary fibrosis. As elevated JNK activation in fibrotic lung fibroblasts contributes to the persistence of the myofibroblast phenotype in pulmonary fibrosis by promoting an autocrine ET-1 loop, targeting the ETA and ETB receptors or constitutive JNK activation by fibrotic lung fibroblasts is likely to be of benefit in combating chronic pulmonary fibrosis.
Figures








References
-
- Abraham, D. J., R. Vancheeswaran, M. R. Dashwood, P. Pantelides, W. Shi-Wen, R. M. du Bois, and C. M. Black. 1997. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am. J. Pathol. 151:831-841. - PMC - PubMed
-
- Abraham, D. J., X. Shiwen, C. M. Black, S. Sa, Y. Xu, and A. Leask. 2000. Tumor necrosis factor alpha suppresses the induction of connective tissue growth factor by transforming growth factor-beta in normal and scleroderma fibroblasts. J. Biol. Chem. 275:15220-15225. - PubMed
-
- Anggard, E. E., R. M. Botting, and J. R. Vane. 1990. Endothelins. Blood Vessels 27:269-281. - PubMed
-
- Badylak, S. F. 2002. The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 13:377-383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
