Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons
- PMID: 16810008
- PMCID: PMC3725035
- DOI: 10.1097/00000542-200607000-00026
Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons
Abstract
Background: Reports of Ca(2+) current I(Ca) loss after injury to peripheral sensory neurons do not discriminate between axotomized and spared neurons. The spinal nerve ligation model separates axotomized from spared neurons innervating the same site. The authors hypothesized that I(Ca) loss is a result of neuronal injury, so they compared axotomized L5 dorsal root ganglion neurons to spared L4 neurons, as well as neurons from rats undergoing skin incision alone.
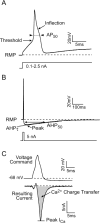
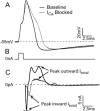
Methods: After behavioral testing, dissociated neurons from L4 and L5 dorsal root ganglia were studied in both current and voltage patch clamp modes. The biophysical consequence of I(Ca) loss on the action potential was confirmed using selective I(Ca) antagonists. Data were grouped into small, medium, and large cells for comparison.
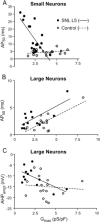
Results: Reduced I(Ca) was predominantly a consequence of axotomy (L5 after spinal nerve ligation) and was most evident in small and medium neurons. ICa losses were associated with action potential prolongation in small and medium cells, whereas the amplitude and duration of after hyperpolarization was reduced in medium and large neurons. Blockade with Ca(2+) channel antagonists showed that action potential prolongation and after hyperpolarization diminution were alike, attributable to the loss of I(Ca).
Conclusion: Axotomy is required for I(Ca) loss. I(Ca) loss correlated with changes in the biophysical properties of sensory neuron membranes during action potential generation, which were due to I(Ca) loss leading to decreased outward Ca(2+)-sensitive K currents. Taken together, these results suggest that neuropathic pain may be mediated, in part, by loss of I(Ca) and the cellular processes dependent on Ca(2+).
Figures


References
-
- Hogan Q. Animal pain models. Reg Anesth Pain Med. 2002;27:385–401. - PubMed
-
- Elmslie KS. Calcium channel blockers in the treatment of disease. J Neurosci Res. 2004;75:733–41. - PubMed
-
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
-
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. - PubMed
-
- Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

