HDAC6-p97/VCP controlled polyubiquitin chain turnover
- PMID: 16810319
- PMCID: PMC1523186
- DOI: 10.1038/sj.emboj.7601210
HDAC6-p97/VCP controlled polyubiquitin chain turnover
Abstract
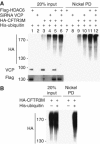
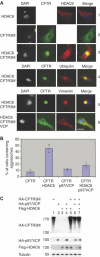
HDAC6 is a unique cytoplasmic deacetylase capable of interacting with ubiquitin. Using a combination of biophysical, biochemical and biological approaches, we have characterized the ubiquitin-binding domain of HDAC6, named ZnF-UBP, and investigated its biological functions. These studies show that the three Zn ion-containing HDAC6 ZnF-UBP domain presents the highest known affinity for ubiquitin monomers and mediates the ability of HDAC6 to negatively control the cellular polyubiquitin chain turnover. We further show that HDAC6-interacting chaperone, p97/VCP, dissociates the HDAC6-ubiquitin complexes and counteracts the ability of HDAC6 to promote the accumulation of polyubiquitinated proteins. We propose that a finely tuned balance of HDAC6 and p97/VCP concentrations determines the fate of ubiquitinated misfolded proteins: p97/VCP would promote protein degradation and ubiquitin turnover, whereas HDAC6 would favour the accumulation of ubiquitinated protein aggregates and inclusion body formation.
Figures



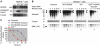

References
-
- Amerik AY, Li SJ, Hochstrasser M (2000) Analysis of the deubiquitinating enzymes of the yeast Saccharomyces cerevisiae. Biol Chem 381: 981–992 - PubMed
-
- Bertos NR, Gilquin B, Chan GK, Yen TJ, Khochbin S, Yang XJ (2004) Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J Biol Chem 279: 48246–48254 - PubMed
-
- Destaing O, Saltel F, Gilquin B, Chabadel A, Khochbin S, Ory S, Jurdic P (2005) A novel Rho–mDia2–HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J Cell Sci 118: 2901–2911 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

