FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCepsilon, B-Raf and S6K2
- PMID: 16810323
- PMCID: PMC1500980
- DOI: 10.1038/sj.emboj.7601198
FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCepsilon, B-Raf and S6K2
Abstract
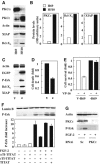
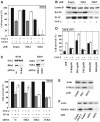
Patients with small cell lung cancer (SCLC) die because of chemoresistance. Fibroblast growth factor-2 (FGF-2) increases the expression of antiapoptotic proteins, XIAP and Bcl-X(L), and triggers chemoresistance in SCLC cells. Here we show that these effects are mediated through the formation of a specific multiprotein complex comprising B-Raf, PKCepsilon and S6K2. S6K1, Raf-1 and other PKC isoforms do not form similar complexes. RNAi-mediated downregulation of B-Raf, PKCepsilon or S6K2 abolishes FGF-2-mediated survival. In contrast, overexpression of PKCepsilon increases XIAP and Bcl-X(L) levels and chemoresistance in SCLC cells. In a tetracycline-inducible system, increased S6K2 kinase activity triggers upregulation of XIAP, Bcl-X(L) and prosurvival effects. However, increased S6K1 kinase activity has no such effect. Thus, S6K2 but not S6K1 mediates prosurvival/chemoresistance signalling.
Figures



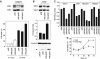
References
-
- Calipel A, Lefevre G, Pouponnot C, Mouriaux F, Eychene A, Mascarelli F (2003) Mutation of B-Raf in human choroidal melanoma cells mediates cell proliferation and transformation through the MEK/ERK pathway. J Biol Chem 278: 42409–42418 - PubMed
-
- Cheng JJ, Wung BS, Chao YJ, Wang DL (2001) Sequential activation of protein kinase C (PKC)-alpha and PKC-epsilon contributes to sustained Raf/ERK1/2 activation in endothelial cells under mechanical strain. J Biol Chem 276: 31368–31375 - PubMed
-
- Dillon TJ, Karpitski V, Wetzel SA, Parker DC, Shaw AS, Stork PJ (2003) Ectopic B-Raf expression enhances extracellular signal-regulated kinase (ERK) signaling in T cells and prevents antigen-presenting cell-induced anergy. J Biol Chem 278: 35940–35949 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

