Poroelastic bulk properties of the tectorial membrane measured with osmotic stress
- PMID: 16815909
- PMCID: PMC1557543
- DOI: 10.1529/biophysj.105.078121
Poroelastic bulk properties of the tectorial membrane measured with osmotic stress
Abstract
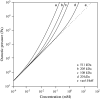
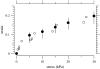
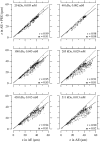
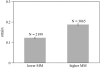
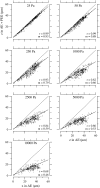
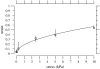
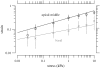
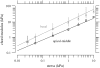
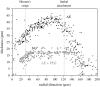
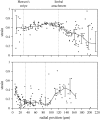
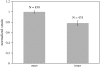
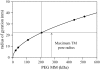
The equilibrium stress-strain relation and the pore radius of the isolated tectorial membrane (TM) of the mouse were determined. Polyethylene glycol (PEG), with molecular mass (MM) in the range 20-511 kDa, added to the TM bathing solution was used to exert an osmotic pressure. Strain on the TM induced by isosmotic PEG solutions of different molecular masses was approximately the same for MM > or = 200 kDa. However, for MM < or = 100 kDa, the TM strain was appreciably smaller. We infer that for the smaller molecular mass, PEG entered the TM and exerted a smaller effective osmotic pressure. The pore radius of the TM was estimated as 22 nm. The equilibrium stress-strain relation of the TM was measured using PEG with a molecular mass of 511 kDa. This relation was nonlinear and was fit with a power function. In the radial cochlear direction, the transverse stiffness of the TM was 20% stiffer in the inner than in the outer region. TM segments from the basal region had a larger transverse stiffness on average compared to sections from the apical-middle region. These measurements provide a quantitative basis for a poroelastic model of the TM.
Figures






Similar articles
-
Basilar membrane and tectorial membrane stiffness in the CBA/CaJ mouse.J Assoc Res Otolaryngol. 2014 Oct;15(5):675-94. doi: 10.1007/s10162-014-0463-y. Epub 2014 May 28. J Assoc Res Otolaryngol. 2014. PMID: 24865766 Free PMC article.
-
Static material properties of the tectorial membrane: a summary.Hear Res. 2003 Jun;180(1-2):11-27. doi: 10.1016/s0378-5955(03)00072-8. Hear Res. 2003. PMID: 12782349 Review.
-
Col11a2 deletion reveals the molecular basis for tectorial membrane mechanical anisotropy.Biophys J. 2009 Jun 3;96(11):4717-24. doi: 10.1016/j.bpj.2009.02.056. Biophys J. 2009. PMID: 19486694 Free PMC article.
-
Dynamic material properties of the tectorial membrane: a summary.Hear Res. 2003 Jun;180(1-2):1-10. doi: 10.1016/s0378-5955(03)00073-x. Hear Res. 2003. PMID: 12782348 Review.
-
Anisotropic Material Properties of Wild-Type and Tectb-/- Tectorial Membranes.Biophys J. 2019 Feb 5;116(3):573-585. doi: 10.1016/j.bpj.2018.12.019. Epub 2019 Jan 5. Biophys J. 2019. PMID: 30665694 Free PMC article.
Cited by
-
Tectorial membrane stiffness gradients.Biophys J. 2007 Sep 15;93(6):2265-76. doi: 10.1529/biophysj.106.094474. Epub 2007 May 11. Biophys J. 2007. PMID: 17496047 Free PMC article.
-
The Tectorial Membrane: Mechanical Properties and Functions.Cold Spring Harb Perspect Med. 2019 Oct 1;9(10):a033514. doi: 10.1101/cshperspect.a033514. Cold Spring Harb Perspect Med. 2019. PMID: 30348837 Free PMC article. Review.
-
Modified protein expression in the tectorial membrane of the cochlea reveals roles for the striated sheet matrix.Biophys J. 2015 Jan 6;108(1):203-10. doi: 10.1016/j.bpj.2014.11.1854. Biophys J. 2015. PMID: 25564867 Free PMC article.
-
Nanoscale Poroelasticity of the Tectorial Membrane Determines Hair Bundle Deflections.Phys Rev Lett. 2019 Jan 18;122(2):028101. doi: 10.1103/PhysRevLett.122.028101. Phys Rev Lett. 2019. PMID: 30720330 Free PMC article.
-
Basilar membrane and tectorial membrane stiffness in the CBA/CaJ mouse.J Assoc Res Otolaryngol. 2014 Oct;15(5):675-94. doi: 10.1007/s10162-014-0463-y. Epub 2014 May 28. J Assoc Res Otolaryngol. 2014. PMID: 24865766 Free PMC article.
References
-
- McGuirt, W. T., S. D. Prasad, A. J. Griffith, H. Kunst, G. E. Green, K. B. Shpargel, C. Runge, C. Huybrechts, R. F. Mueller, E. Lynch, M. C. King, H. G. Brunner, C. Cremers, M. Takanosu, S. W. Li, M. Arita, R. Mayne, D. J. Prockop, G. Van Camp, and R. Smith. 1999. Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13). Nat. Genet. 23:413–419. - PubMed
-
- Legan, P. K., V. A. Lukashkina, R. J. Goodyear, M. Kossl, I. J. Russell, and G. P. Richardson. 2000. A targeted deletion of α-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron. 28:273–285. - PubMed
-
- Legan, P. K., V. A. Lukashkina, R. J. Goodyear, A. N. Lukashkin, K. Verhoeven, G. V. Camp, I. J. Russell, and G. P. Richardson. 2005. A deafness mutation isolates a second role for the tectorial membrane in hearing. Nat. Neurosci. 8:1035–1042. - PubMed
-
- Simmler, M. C., M. Cohen-Salmon, A. El-Amraoui, L. Guillaud, J. C. Benichou, C. Petit, and J. J. Panthier. 2000. Targeted disruption of otog results in deafness and severe imbalance. Nat. Genet. 24:139–143. - PubMed
-
- Davis, H. 1958. A mechano-electrical theory of cochlear action. Ann. Otol. Rhinol. Laryngol. 67:789–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

