Induction of differentiation in the shoot apical meristem by transient overexpression of a retinoblastoma-related protein
- PMID: 16815954
- PMCID: PMC1533950
- DOI: 10.1104/pp.106.083022
Induction of differentiation in the shoot apical meristem by transient overexpression of a retinoblastoma-related protein
Abstract
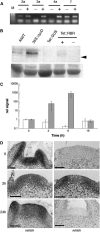
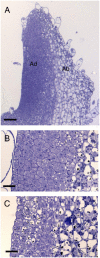
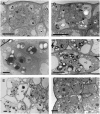
The shoot apical meristem contains cells that undergo continual growth and division to generate the building blocks for the aerial portion of the plant. As cells leave the meristem, they undergo differentiation to form specific cell types. Most notably, heterotrophic cells of the meristem rapidly gain autotrophic capability by synthesis and assembly of components of the chloroplast. At the same time, cells undergo enlargement via vacuolation. Despite significant advances in the characterization of transcriptional networks involved in meristem maintenance and leaf determination, our understanding of the actual mechanism of meristem cell differentiation remains very limited. Using a microinduction technique, we show that local, transient overexpression of a retinoblastoma-related (RBR) protein in the shoot apical meristem is sufficient to trigger cells in the meristem to undergo the initial stages of differentiation. Taken together with recent data showing that RBR protein plays a key role in restricting stem cell differentiation in the root apical meristem, our data contribute to an emerging picture of RBR proteins as a central part of the mechanism controlling meristem cell differentiation.
Figures



Similar articles
-
Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers.Nature. 2007 Apr 12;446(7137):811-4. doi: 10.1038/nature05703. Nature. 2007. PMID: 17429400
-
AtCDC5 regulates the G2 to M transition of the cell cycle and is critical for the function of Arabidopsis shoot apical meristem.Cell Res. 2007 Sep;17(9):815-28. doi: 10.1038/cr.2007.71. Cell Res. 2007. PMID: 17768399
-
Auxin modulates the transition from the mitotic cycle to the endocycle in Arabidopsis.Development. 2010 Jan;137(1):63-71. doi: 10.1242/dev.035840. Development. 2010. PMID: 20023161
-
Stem cell regulation in the Arabidopsis shoot apical meristem.Curr Opin Plant Biol. 2005 Dec;8(6):582-6. doi: 10.1016/j.pbi.2005.09.010. Epub 2005 Sep 23. Curr Opin Plant Biol. 2005. PMID: 16183326 Review.
-
Coordination of cell proliferation and cell fate decisions in the angiosperm shoot apical meristem.Bioessays. 2002 Jan;24(1):27-37. doi: 10.1002/bies.10020. Bioessays. 2002. PMID: 11782948 Review.
Cited by
-
pRb-E2F signaling in life of mesenchymal stem cells: Cell cycle, cell fate, and cell differentiation.Genes Dis. 2014 Sep 30;1(2):174-187. doi: 10.1016/j.gendis.2014.09.007. eCollection 2014 Dec. Genes Dis. 2014. PMID: 30258863 Free PMC article. Review.
-
Retinoblastoma protein is essential for early meiotic events in Arabidopsis.EMBO J. 2011 Feb 16;30(4):744-55. doi: 10.1038/emboj.2010.344. Epub 2011 Jan 7. EMBO J. 2011. PMID: 21217641 Free PMC article.
-
The retinoblastoma tumor suppressor and stem cell biology.Genes Dev. 2012 Jul 1;26(13):1409-20. doi: 10.1101/gad.193730.112. Genes Dev. 2012. PMID: 22751497 Free PMC article. Review.
-
Understanding of Leaf Development-the Science of Complexity.Plants (Basel). 2013 Jun 25;2(3):396-415. doi: 10.3390/plants2030396. Plants (Basel). 2013. PMID: 27137383 Free PMC article. Review.
-
MEDIATOR SUBUNIT17 is required for transcriptional optimization of root system architecture in Arabidopsis.Plant Physiol. 2023 May 31;192(2):1548-1568. doi: 10.1093/plphys/kiad129. Plant Physiol. 2023. PMID: 36852886 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

