Interaction of two hereditary spastic paraplegia gene products, spastin and atlastin, suggests a common pathway for axonal maintenance
- PMID: 16815977
- PMCID: PMC1502289
- DOI: 10.1073/pnas.0510863103
Interaction of two hereditary spastic paraplegia gene products, spastin and atlastin, suggests a common pathway for axonal maintenance
Abstract
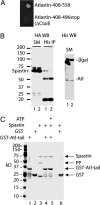
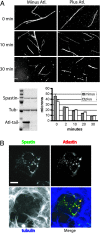
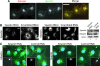
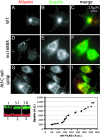
Hereditary spastic paraplegia (HSP) is a neurodegenerative disorder that is characterized by retrograde axonal degeneration that primarily affects long spinal neurons. The disease is clinically heterogeneous, and there are >20 genetic loci identified. Here, we show a physical interaction between spastin and atlastin, two autosomal dominant HSP gene products. Spastin encodes a microtubule (MT)-severing AAA ATPase (ATPase associated with various activities), and atlastin encodes a Golgi-localized integral membrane protein GTPase. Atlastin does not regulate the enzymatic activity of spastin. We also identified a clinical mutation in atlastin outside of the GTPase domain that prevents interaction with spastin in cells. Therefore, we hypothesize that failure of appropriate interaction between these two HSP gene products may be pathogenetically relevant. These data indicate that at least a subset of HSP genes may define a cellular biological pathway that is important in axonal maintenance.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Fink J. K. Ann. Neurol. 2002;51:669–672. - PubMed
-
- Casari G., De Fusco M., Ciarmatori S., Zeviani M., Mora M., Fernandez P., De Michele G., Filla A., Cocozza S., Marconi R., et al. Cell. 1998;93:973–983. - PubMed
-
- Hazan J., Fonknechten N., Mavel D., Paternotte C., Samson D., Artiguenave F., Davoine C. S., Cruaud C., Durr A., Wincker P., et al. Nat. Genet. 1999;23:296–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

