The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway
- PMID: 16815997
- PMCID: PMC1522081
- DOI: 10.1101/gad.1411206
The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway
Abstract
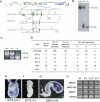
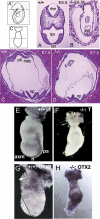
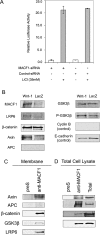
MACF1 (microtubule actin cross-linking factor 1) is a multidomain protein that can associate with microfilaments and microtubules. We found that MACF1 was highly expressed in neuronal tissues and the foregut of embryonic day 8.5 (E8.5) embryos and the head fold and primitive streak of E7.5 embryos. MACF1(-/-) mice died at the gastrulation stage and displayed developmental retardation at E7.5 with defects in the formation of the primitive streak, node, and mesoderm. This phenotype was similar to Wnt-3(-/-) and LRP5/6 double-knockout embryos. In the absence of Wnt, MACF1 associated with a complex that contained Axin, beta-catenin, GSK3beta, and APC. Upon Wnt stimulation, MACF1 appeared to be involved in the translocation and subsequent binding of the Axin complex to LRP6 at the cell membrane. Reduction of MACF1 with small interfering RNA decreased the amount of beta-catenin in the nucleus, and led to an inhibition of Wnt-induced TCF/beta-catenin-dependent transcriptional activation. Similar results were obtained with a dominant-negative MACF1 construct that contained the Axin-binding region. Reduction of MACF1 in Wnt-1-expressing P19 cells resulted in decreased T (Brachyury) gene expression, a DNA-binding transcription factor that is a direct target of Wnt/beta-catenin signaling and required for mesoderm formation. These results suggest a new role of MACF1 in the Wnt signaling pathway.
Figures
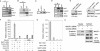


References
-
- Arnold S.J., Stappert J., Bauer A., Kispert A., Herrmann B.G., Kemler R. Brachyury is a target gene of the Wnt/β-catenin signaling pathway. Mech. Dev. 2000;91:249–258. - PubMed
-
- Beddington R.S., Robertson E.J. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. - PubMed
-
- Chen H.J., Hwong C.L., Wang C.H., Hwang J. Degradation of DNA topoisomerase I by a novel trypsin-like serine protease in proliferating human T lymphocytes. J. Biol. Chem. 2000;275:13109–13117. - PubMed
-
- Cliffe A., Hamada F., Bienz M. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr. Biol. 2003;13:960–966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
