The Brucella abortus cyclic beta-1,2-glucan virulence factor is substituted with O-ester-linked succinyl residues
- PMID: 16816173
- PMCID: PMC1539967
- DOI: 10.1128/JB.00086-06
The Brucella abortus cyclic beta-1,2-glucan virulence factor is substituted with O-ester-linked succinyl residues
Abstract
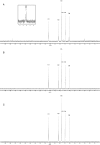
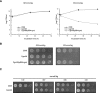
Brucella periplasmic cyclic beta-1,2-glucan plays an important role during bacterium-host interaction. Nuclear magnetic resonance spectrometry analysis, thin-layer chromatography, and DEAE-Sephadex chromatography were used to characterize Brucella abortus cyclic glucan. In the present study, we report that a fraction of B. abortus cyclic beta-1,2-glucan is substituted with succinyl residues, which confer anionic character on the cyclic beta-1,2-glucan. The oligosaccharide backbone is substituted at C-6 positions with an average of two succinyl residues per glucan molecule. This O-ester-linked succinyl residue is the only substituent of Brucella cyclic glucan. A B. abortus open reading frame (BAB1_1718) homologous to Rhodobacter sphaeroides glucan succinyltransferase (OpgC) was identified as the gene encoding the enzyme responsible for cyclic glucan modification. This gene was named cgm for cyclic glucan modifier and is highly conserved in Brucella melitensis and Brucella suis. Nucleotide sequencing revealed that B. abortus cgm consists of a 1,182-bp open reading frame coding for a predicted membrane protein of 393 amino acid residues (42.7 kDa) 39% identical to Rhodobacter sphaeroides succinyltransferase. cgm null mutants in B. abortus strains 2308 and S19 produced neutral glucans without succinyl residues, confirming the identity of this protein as the cyclic-glucan succinyltransferase enzyme. In this study, we demonstrate that succinyl substituents of cyclic beta-1,2-glucan of B. abortus are necessary for hypo-osmotic adaptation. On the other hand, intracellular multiplication and mouse spleen colonization are not affected in cgm mutants, indicating that cyclic-beta-1,2-glucan succinylation is not required for virulence and suggesting that no low-osmotic stress conditions must be overcome during infection.
Figures






References
-
- Altabe, S. G., P. Talaga, J. M. Wieruszeski, G. Lippens, R. Ugalde, and J. P. Bohin. 1998. Periplasmic glucans of Azospirillum brasilense, p. 390. In C. Elmerich, A. Kondorosi, and W. E. Newton (ed.), Biological nitrogen fixation for the 21st century. Kluwer Academic Publishers, Dordrecht, The Netherlands.
-
- Arellano-Reynoso, B., N. Lapaque, S. Salcedo, G. Briones, A. E. Ciocchini, R. Ugalde, E. Moreno, I. Moriyon, and J. P. Gorvel. 2005. Cyclic beta-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nat. Immunol. 6:618-625. - PubMed
-
- Batley, M., J. W. Redmond, and A. J. Wicken. 1987. Nuclear magnetic resonance spectra of lipoteichoic acid. Biochim. Biophys. Acta 901:127-137. - PubMed
-
- Bohin, J. P. 2000. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol. Lett. 186:11-19. - PubMed
-
- Bohin, J. P., and E. P. Kennedy. 1984. Regulation of the synthesis of membrane-derived oligosaccharides in Escherichia coli. Assay of phosphoglycerol transferase I in vivo. J. Biol. Chem. 259:8388-8393. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

