Roles of the intramolecular disulfide bridge in MotX and MotY, the specific proteins for sodium-driven motors in Vibrio spp
- PMID: 16816206
- PMCID: PMC1539959
- DOI: 10.1128/JB.00187-06
Roles of the intramolecular disulfide bridge in MotX and MotY, the specific proteins for sodium-driven motors in Vibrio spp
Abstract
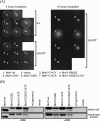
The proteins PomA, PomB, MotX, and MotY are essential for the motor function of Na+-driven flagella in Vibrio spp. Both MotY and MotX have the two cysteine residues (one of which is in a conserved tetrapeptide [CQLV]) that are inferred to form an intramolecular disulfide bond. The cysteine mutants of MotY prevented the formation of an intramolecular disulfide bond, which is presumably important for protein stability. Disruption of the disulfide bridge in MotX by site-directed mutagenesis resulted in increased instability, which did not, however, affect the motility of the cells. These lines of evidence suggest that the intramolecular disulfide bonds are involved in the stability of both proteins, but only MotY requires the intramolecular bridge for proper function.
Figures
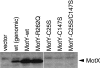



Similar articles
-
MotX and MotY, specific components of the sodium-driven flagellar motor, colocalize to the outer membrane in Vibrio alginolyticus.Mol Microbiol. 2002 Oct;46(1):125-34. doi: 10.1046/j.1365-2958.2002.03142.x. Mol Microbiol. 2002. PMID: 12366836
-
Cloning and characterization of motX, a Vibrio alginolyticus sodium-driven flagellar motor gene.J Biochem. 2001 Dec;130(6):879-84. doi: 10.1093/oxfordjournals.jbchem.a003061. J Biochem. 2001. PMID: 11726290
-
Interactions of MotX with MotY and with the PomA/PomB sodium ion channel complex of the Vibrio alginolyticus polar flagellum.J Biol Chem. 2005 Jul 8;280(27):25659-64. doi: 10.1074/jbc.M500263200. Epub 2005 May 2. J Biol Chem. 2005. PMID: 15866878
-
Sodium-driven motor of the polar flagellum in marine bacteria Vibrio.Genes Cells. 2011 Oct;16(10):985-99. doi: 10.1111/j.1365-2443.2011.01545.x. Epub 2011 Sep 5. Genes Cells. 2011. PMID: 21895888 Review.
-
Pathways of disulfide bond formation in Escherichia coli.Int J Biochem Cell Biol. 2006;38(7):1050-62. doi: 10.1016/j.biocel.2005.12.011. Epub 2006 Jan 11. Int J Biochem Cell Biol. 2006. PMID: 16446111 Review.
Cited by
-
The FlgT Protein Is Involved in Aeromonas hydrophila Polar Flagella Stability and Not Affects Anchorage of Lateral Flagella.Front Microbiol. 2016 Jul 26;7:1150. doi: 10.3389/fmicb.2016.01150. eCollection 2016. Front Microbiol. 2016. PMID: 27507965 Free PMC article.
-
MotX and MotY are required for flagellar rotation in Shewanella oneidensis MR-1.J Bacteriol. 2009 Aug;191(16):5085-93. doi: 10.1128/JB.00206-09. Epub 2009 Jun 5. J Bacteriol. 2009. PMID: 19502394 Free PMC article.
-
Crystallization and preliminary X-ray analysis of MotY, a stator component of the Vibrio alginolyticus polar flagellar motor.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007 Feb 1;63(Pt 2):89-92. doi: 10.1107/S1744309106055850. Epub 2007 Jan 17. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007. PMID: 17277446 Free PMC article.
-
Isolation of basal bodies with C-ring components from the Na+-driven flagellar motor of Vibrio alginolyticus.J Bacteriol. 2010 Jan;192(1):375-8. doi: 10.1128/JB.01121-09. J Bacteriol. 2010. PMID: 19880601 Free PMC article.
-
The flagellar basal body-associated protein FlgT is essential for a novel ring structure in the sodium-driven Vibrio motor.J Bacteriol. 2010 Nov;192(21):5609-15. doi: 10.1128/JB.00720-10. Epub 2010 Aug 20. J Bacteriol. 2010. PMID: 20729351 Free PMC article.
References
-
- Atsumi, T., Y. Maekawa, H. Tokuda, and Y. Imae. 1992. Amiloride at pH 7.0 inhibits the Na+-driven flagellar motors of Vibrio alginolyticus but allows the cell growth. FEBS Lett. 314:114-116. - PubMed
-
- Bardwell, J. C., K. McGovern, and J. Beckwith. 1991. Identification of a protein required for disulfide bond formation in vivo. Cell 67:581-589. - PubMed
-
- Bartolome, B., Y. Jubete, E. Martinez, and F. de la Cruz. 1991. Construction and properties of a family of pACY184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75-78. - PubMed
-
- Berg, H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19-54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

