Obligatory role for interleukin-13 in obstructive lesion development in airway allografts
- PMID: 16816360
- PMCID: PMC1698762
- DOI: 10.2353/ajpath.2006.050975
Obligatory role for interleukin-13 in obstructive lesion development in airway allografts
Abstract
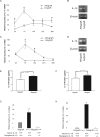
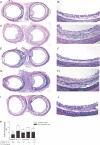
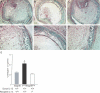
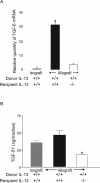
The pathogenesis of bronchiolitis obliterans (BO), a common and devastating obliterative disorder of small airways following lung transplantation, remains poorly understood. Lesions are characterized in their early stages by lymphocyte influx that evolves into dense fibrotic infiltrates. Airway specimens taken from patients with histological BO revealed infiltrating myofibroblasts, which strongly expressed the signaling chain of the high affinity interleukin-13 (IL-13) receptor IL-13Ralpha1. Because IL-13 has proinflammatory and profibrotic actions, a contributory role for IL-13 in BO development was examined using murine models of orthotopic and heterotopic tracheal transplantation. Compared with airway isografts, allografts exhibited a significant increase in relative IL-13 mRNA and protein levels. Allogeneic tracheas transplanted into IL-13-deficient mice were protected from BO in both transplant models. Flow cytometric analysis of orthotopic transplant tissue digests revealed markedly fewer infiltrating mononuclear phagocytes and CD3(+) T lymphocytes in IL-13-deficient recipients. Furthermore, protection from luminal obliteration, collagen deposition, and myofibroblast infiltration was observed in heterotopic airways transplanted into the IL-13(-/-) recipients. Transforming growth factor-beta1 expression was significantly decreased in tracheal allografts into IL-13(-/-) recipients, compared to wild-type counterparts. These human and murine data implicate IL-13 as a critical effector cytokine driving cellular recruitment and subsequent fibrosis in clinical and ex-perimental BO.
Figures




Similar articles
-
[Establishment of obliterative bronchiolitis in allo-trachea transplant model of rat and detection of its pathogenesis preliminarily].Zhonghua Wai Ke Za Zhi. 2007 Feb 15;45(4):262-6. Zhonghua Wai Ke Za Zhi. 2007. PMID: 17502025 Chinese.
-
Rapamycin prevents bronchiolitis obliterans through increasing infiltration of regulatory B cells in a murine tracheal transplantation model.J Thorac Cardiovasc Surg. 2016 Feb;151(2):487-96.e3. doi: 10.1016/j.jtcvs.2015.08.116. Epub 2015 Sep 7. J Thorac Cardiovasc Surg. 2016. PMID: 26481278 Free PMC article.
-
Orthotopic and heterotopic tracheal transplantation model in studying obliterative bronchiolitis.Transpl Immunol. 2013 Jun;28(4):170-5. doi: 10.1016/j.trim.2013.04.006. Epub 2013 Apr 22. Transpl Immunol. 2013. PMID: 23619376
-
Cyclosporine A drives a Th17- and Th2-mediated posttransplant obliterative airway disease.Am J Transplant. 2013 Mar;13(3):611-20. doi: 10.1111/ajt.12067. Epub 2013 Jan 17. Am J Transplant. 2013. PMID: 23331973
-
Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development.J Heart Lung Transplant. 2010 Dec;29(12):1405-14. doi: 10.1016/j.healun.2010.07.005. J Heart Lung Transplant. 2010. PMID: 20920842 Free PMC article.
Cited by
-
Prostaglandin E2 as an inhibitory modulator of fibrogenesis in human lung allografts.Am J Respir Crit Care Med. 2012 Jan 1;185(1):77-84. doi: 10.1164/rccm.201105-0834OC. Am J Respir Crit Care Med. 2012. PMID: 21940790 Free PMC article.
-
Mechanistic Target of Rapamycin Complex 1 (mTORC1) and mTORC2 as Key Signaling Intermediates in Mesenchymal Cell Activation.J Biol Chem. 2016 Mar 18;291(12):6262-71. doi: 10.1074/jbc.M115.672170. Epub 2016 Jan 11. J Biol Chem. 2016. PMID: 26755732 Free PMC article.
-
Bronchiolitis obliterans syndrome: the Achilles' heel of lung transplantation.Semin Respir Crit Care Med. 2013 Jun;34(3):336-51. doi: 10.1055/s-0033-1348467. Epub 2013 Jul 2. Semin Respir Crit Care Med. 2013. PMID: 23821508 Free PMC article. Review.
-
Phenotypic and functional changes of cytotoxic CD56pos natural T cells determine outcome of acute hepatitis C virus infection.J Virol. 2007 Sep;81(17):9292-8. doi: 10.1128/JVI.00834-07. Epub 2007 Jun 6. J Virol. 2007. PMID: 17553896 Free PMC article.
-
Lung transplantation: infection, inflammation, and the microbiome.Semin Immunopathol. 2011 Mar;33(2):135-56. doi: 10.1007/s00281-011-0249-9. Epub 2011 Jan 27. Semin Immunopathol. 2011. PMID: 21271250 Review.
References
-
- Boehler A, Kesten S, Weder W, Speich R. Bronchiolitis obliterans after lung transplantation: a review. Chest. 1998;114:1411–1426. - PubMed
-
- Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. - PubMed
-
- Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21:271–281. - PubMed
-
- Boehler A, Chamberlain D, Kesten S, Slutsky AS, Liu M, Keshavjee S. Lymphocytic airway infiltration as a precursor to fibrous obliteration in a rat model of bronchiolitis obliterans. Transplantation. 1997;64:311–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

