Pathogenic role for virus-specific CD4 T cells in mice with coronavirus-induced acute encephalitis
- PMID: 16816374
- PMCID: PMC1698761
- DOI: 10.2353/ajpath.2006.051308
Pathogenic role for virus-specific CD4 T cells in mice with coronavirus-induced acute encephalitis
Abstract
Acute viral encephalitis is believed to result from direct virus destruction of infected cells and from virus-induced host immune response, but the relative contribution of each remains largely unknown. For example, C57BL/6 (B6) mice infected with mouse hepatitis virus (JHM strain, JHMV) develop severe encephalitis, with death occurring within 7 days. Here, we show that the host response to a single JHMV-specific immunodominant CD4 T-cell epitope is critical for severe disease. We engineered a recombinant JHMV with mutations in the immunodominant CD4 T-cell epitope (rJ.M(Y135Q)). Infection of naïve B6 mice with this virus resulted in mild disease with no mortality. However, introduction of a CD4 T-cell epitope from Listeria monocytogenes into rJ.M(Y135Q) generated a highly virulent virus. The decrease in disease severity was not due to a switch from Th1 to Th2 predominance in rJ.M(Y135Q)-infected mice, an effect on CD8 T-cell function, or differential expression of tumor necrosis factor-alpha by JHMV-specific CD4 T cells. These results show that the response to a single virus-specific CD4 T-cell epitope may contribute to a pathogenic host response in the setting of acute viral disease and that abrogation of this response ameliorates clinical disease without diminishing virus clearance.
Figures
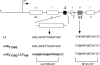





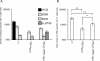
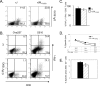

References
-
- Stohlman SA, Bergmann CC, Perlman S. Mouse Hepatitis Virus. Ahmed R, Chen I, editors. New York,: John Wiley & Sons, Ltd.,; 1998:pp 537–557.
-
- Barnett E, Perlman S. The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology. 1993;194:185–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

