Role of the integrin-binding protein osteopontin in lymphatic metastasis of breast cancer
- PMID: 16816376
- PMCID: PMC1698777
- DOI: 10.2353/ajpath.2006.051152
Role of the integrin-binding protein osteopontin in lymphatic metastasis of breast cancer
Abstract
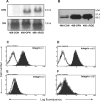
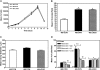
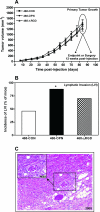
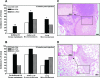
Although a primary route of breast cancer metastasis is believed to be via lymphatics, the molecular factors involved are poorly understood. We hypothesized that one such factor may be the integrin-binding protein osteopontin (OPN), and we investigated this clinically and experimentally. In breast cancer patients undergoing sentinel lymph node biopsy, OPN levels were significantly higher in lymph node metastases than in the primary tumor (P < 0.001). To test the functional contribution of OPN to lymphatic metastasis and to determine whether the RGD (Arg-Gly-Asp) integrin-binding sequence of OPN is important for this process, we transfected wild-type OPN or mutant OPN (lacking the RGD sequence) into MDA-MB-468 human breast cancer cells. In vitro, cells overexpressing OPN demonstrated increased anchorage-independent growth in soft agar (P = 0.001) and increased RGD-dependent adhesion (P = 0.045). Following mammary fat pad injection of nude mice, cells overexpressing OPN showed increased lymphovascular invasion, lymph node metastases, and lung micrometastases at earlier time points (P = 0.024). Loss of the RGD region partially abrogated this effect in the lymphatics (P = 0.038). These novel findings indicate that OPN is a key molecular player involved in lymphatic metastasis of breast cancer, potentially by affecting RGD-mediated adhesive interactions and by enhancing the establishment/persistence of tumor cells in the lymphatics.
Figures

References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
-
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. - PubMed
-
- Fidler IJ. 7th Jan Waldenstrom Lecture. The biology of human cancer metastasis. Acta Oncol. 1991;30:668–675. - PubMed
-
- MacDonald IC, Groom AC, Chambers AF. Cancer spread and micrometastasis development: quantitative approaches for in vivo models. BioEssays. 2002;24:885–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

