Protease-activated receptor-2 activation in gastric cancer cells promotes epidermal growth factor receptor trans-activation and proliferation
- PMID: 16816379
- PMCID: PMC1698759
- DOI: 10.2353/ajpath.2006.050841
Protease-activated receptor-2 activation in gastric cancer cells promotes epidermal growth factor receptor trans-activation and proliferation
Abstract
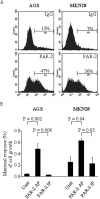
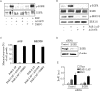
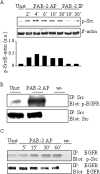
Dysregulated epidermal growth factor receptor (EGFR) signaling is involved in gastric cancer (GC) cell growth. However, the mechanism that sustains EGFR signaling in GC remains unknown. Since protease-activated receptor-2 (PAR-2), a G protein-coupled receptor, has been shown to trans-activate EGFR in several cell types, we examined the role of PAR-2 in GC. We show here that in vitro activation of PAR-2 enhances the growth of two GC cell lines, AGS and MKN28. In both these cell lines, PAR-2 trans-activated EGFR and inhibition of EGFR tyrosine kinase activity by AG1478 or specific EGFR siRNA completely prevented PAR-2-driven proliferation. Antibody blockade of EGF-like ligands to EGFR did not modify EGFR signaling or cell growth induced by PAR-2 activation. In contrast, PAR-2 promoted Src activation and interaction of this kinase with EGFR. In support of this, inhibition of Src kinase activity by PP1 or siRNA blocked PAR-2-induced EGFR signaling cascade and cell growth. Finally, PAR-2 was detectable in both normal and GC specimens, but its expression was more pronounced in GC than controls and correlated with activated EGFR. These data show that PAR-2 is overexpressed in GC and suggest a role of PAR-2 in EGFR trans-activation and cell growth.
Figures




References
-
- Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–315. - PubMed
-
- Tokunaga A, Onda M, Okuda T, Teramoto T, Fujita I, Mizutani T, Kiyama T, Yoshiyuki T, Nishi K, Matsukura N. Clinical significance of epidermal growth factor (EGF), EGF receptor, and c-erbB-2 in human gastric cancer. Cancer. 1995;75(suppl):1418–1425. - PubMed
-
- Naef M, Yokoyama M, Friess H, Büchler MW, Korc M. Co-expression of heparin-binding EGF-like growth factor and related peptides in human gastric carcinoma. Int J Cancer. 1996;66:315–321. - PubMed
-
- Seaki T, Salomon D, Normanno N, Johnson G, Gullick WJ, Mandai K, Moriwaki S, Takashima S, Kuniyasu M, Tahara E, Kawami H, Nishiyama M, Toge T. Immunohistochemical detection of cripto-1, amphiregulin and transforming growth factor alpha in human gastric carcinomas and intestinal metaplasias. Int J Oncol. 1994;5:215–223. - PubMed
-
- Daub H, Weiss U, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

