Involvement of endothelial CD44 during in vivo angiogenesis
- PMID: 16816384
- PMCID: PMC1698758
- DOI: 10.2353/ajpath.2006.060206
Involvement of endothelial CD44 during in vivo angiogenesis
Erratum in
- Am J Pathol. 2006 Nov;169(5):1899
Abstract
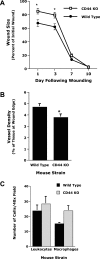
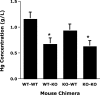
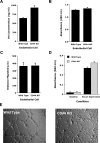
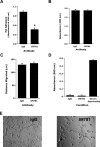
CD44, a cell-surface receptor for hyaluronan, has been implicated in endothelial cell functions, but its role in the formation of blood vessels in vivo has not been established. In CD44-null mice, vascularization of Matrigel implants and tumor and wound angiogenesis were inhibited. Leukocyte accumulation during tumor growth and wound healing in wild-type and CD44-null mice were comparable, and reconstitution of CD44-null mice with wild-type bone marrow did not restore the wild-type phenotype, suggesting that impairments in angiogenesis in CD44-deficient mice are due to the loss of endothelial CD44. Although the cell proliferation, survival, and wound-induced migration of CD44-null endothelial cells were intact, these cells were impaired in their in vitro ability to form tubes. Nascent vessels in Matrigel implants from CD44-null mice demonstrated irregular luminal surfaces characterized by retracted cells and thinned endothelia. Further, an anti-CD44 antibody that disrupted in vitro tube formation induced hemorrhage around Matrigel implants, suggesting that antagonism of endothelial CD44 undermined the integrity of the endothelium of nascent vessels. These data establish a role for CD44 during in vivo angiogenesis and suggest that CD44 may contribute to the organization and/or stability of developing endothelial tubular networks.
Figures




References
-
- Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. - PubMed
-
- Savani RC, DeLisser Hyaluronan and its receptors in lung health and disease. Garg HG, Roughley PJ, Hales CA, editors. New York: Marcel Dekker, Inc.,; Proteoglycans in Lung Disease. 2002:pp 73–106.
-
- Rooney P, Kumar S, Ponting J, Wang M. The role of hyaluronan in tumour neovascularization. Int J Cancer. 1995;60:632–636. - PubMed
-
- West DC, Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res. 1989;183:179–196. - PubMed
-
- Feinberg RN, Beebe DC. Hyaluronate in vasculogenesis. Science. 1983;220:1177–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

