Melanotic mutants in Drosophila: pathways and phenotypes
- PMID: 16816412
- PMCID: PMC1569781
- DOI: 10.1534/genetics.106.061978
Melanotic mutants in Drosophila: pathways and phenotypes
Abstract
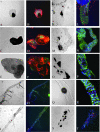
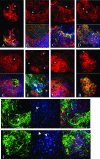
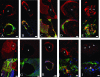
Mutations in >30 genes that regulate different pathways and developmental processes are reported to cause a melanotic phenotype in larvae. The observed melanotic masses were generally linked to the hemocyte-mediated immune response. To investigate whether all black masses are associated with the cellular immune response, we characterized melanotic masses from mutants in 14 genes. We found that the melanotic masses can be subdivided into melanotic nodules engaging the hemocyte-mediated encapsulation and into melanizations that are not encapsulated by hemocytes. With rare exception, the encapsulation is carried out by lamellocytes. Encapsulated nodules are found in the hemocoel or in association with the lymph gland, while melanizations are located in the gut, salivary gland, and tracheae. In cactus mutants we found an additional kind of melanized mass containing various tissues. The development of these tissue agglomerates is dependent on the function of the dorsal gene. Our results show that the phenotype of each mutant not only reflects its connection to a particular genetic pathway but also points to the tissue-specific role of the individual gene.
Figures
Similar articles
-
A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis.Development. 1998 May;125(10):1909-20. doi: 10.1242/dev.125.10.1909. Development. 1998. PMID: 9550723
-
Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila.Genetics. 2004 Mar;166(3):1343-56. doi: 10.1534/genetics.166.3.1343. Genetics. 2004. PMID: 15082553 Free PMC article.
-
Genetic Screen in Drosophila Larvae Links ird1 Function to Toll Signaling in the Fat Body and Hemocyte Motility.PLoS One. 2016 Jul 28;11(7):e0159473. doi: 10.1371/journal.pone.0159473. eCollection 2016. PLoS One. 2016. PMID: 27467079 Free PMC article.
-
Overexpression of jumu induces melanotic nodules by activating Toll signaling in Drosophila.Insect Biochem Mol Biol. 2016 Oct;77:31-38. doi: 10.1016/j.ibmb.2016.08.002. Epub 2016 Aug 6. Insect Biochem Mol Biol. 2016. PMID: 27507244
-
Pigmentation and behavior: potential association through pleiotropic genes in Drosophila.Genes Genet Syst. 2013;88(3):165-74. doi: 10.1266/ggs.88.165. Genes Genet Syst. 2013. PMID: 24025245 Review.
Cited by
-
A new BCR-ABL1 Drosophila model as a powerful tool to elucidate the pathogenesis and progression of chronic myeloid leukemia.Haematologica. 2019 Apr;104(4):717-728. doi: 10.3324/haematol.2018.198267. Epub 2018 Nov 8. Haematologica. 2019. PMID: 30409797 Free PMC article.
-
The Biology of SUMO-Targeted Ubiquitin Ligases in Drosophila Development, Immunity, and Cancer.J Dev Biol. 2018 Jan 1;6(1):2. doi: 10.3390/jdb6010002. J Dev Biol. 2018. PMID: 29615551 Free PMC article. Review.
-
Drosophila poly suggests a novel role for the Elongator complex in insulin receptor-target of rapamycin signalling.Open Biol. 2012 Jan;2(1):110031. doi: 10.1098/rsob.110031. Open Biol. 2012. PMID: 22645656 Free PMC article.
-
Two Isoforms of serpent Containing Either One or Two GATA Zinc Fingers Provide Functional Diversity During Drosophila Development.Front Cell Dev Biol. 2022 Feb 1;9:795680. doi: 10.3389/fcell.2021.795680. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35178397 Free PMC article.
-
Proteasome α6 Subunit Negatively Regulates the JAK/STAT Pathway and Blood Cell Activation in Drosophila melanogaster.Front Immunol. 2021 Dec 22;12:729631. doi: 10.3389/fimmu.2021.729631. eCollection 2021. Front Immunol. 2021. PMID: 35003057 Free PMC article.
References
-
- Agaisse, H., and N. Perrimon, 2004. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 198: 72–82. - PubMed
-
- Barigozzi, C., 1958. Melanotic tumors in Drosophila. J. Cell. Physiol. 52: 371–381. - PubMed
-
- Becker, S., A. Gehrsitz, P. Bork, S. Buchner and E. Buchner, 2001. The black-pearl gene of Drosophila defines a novel conserved protein family and is required for larval growth and survival. Gene 262: 15–22. - PubMed
-
- Belvin, M. P., and K. V. Anderson, 1996. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu. Rev. Cell Dev. Biol. 12: 393–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

