An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2
- PMID: 16817851
- PMCID: PMC1893115
- DOI: 10.1111/j.1742-4658.2006.05302.x
An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2
Erratum in
- FEBS J. 2006 Sep;273(17):4129
Abstract
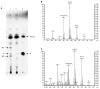
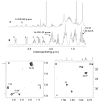
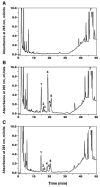
We report an alternative, hydroxylating pathway for the metabolism of vitamin D2 in a cytochrome P450 side chain cleavage (P450scc; CYP11A1) reconstituted system. NMR analyses identified solely 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2 derivatives. 20-Hydroxyvitamin D2 was produced at a rate of 0.34 mol x min(-1) x mol(-1) P450scc, and 17,20-dihydroxyvitamin D2 was produced at a rate of 0.13 mol x min(-1) x mol(-1). In adrenal mitochondria, vitamin D2 was metabolized to six monohydroxy products. Nevertheless, aminoglutethimide (a P450scc inhibitor) inhibited this adrenal metabolite formation. Initial testing of metabolites for biological activity showed that, similar to vitamin D2, 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2 inhibited DNA synthesis in human epidermal HaCaT keratinocytes, although to a greater degree. 17,20-Dihydroxyvitamin D2 stimulated transcriptional activity of the involucrin promoter, again to a significantly greater extent than vitamin D2, while the effect of 20-hydroxyvitamin D2 was statistically insignificant. Thus, P450scc can metabolize vitamin D2 to generate novel products, with intrinsic biological activity (at least in keratinocytes).
Figures

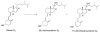
Similar articles
-
Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells.Drug Metab Dispos. 2011 Sep;39(9):1577-88. doi: 10.1124/dmd.111.040071. Epub 2011 Jun 15. Drug Metab Dispos. 2011. PMID: 21677063 Free PMC article.
-
Metabolism of vitamin d2 to 17,20,24-trihydroxyvitamin d2 by cytochrome p450scc (CYP11A1).Drug Metab Dispos. 2009 Apr;37(4):761-7. doi: 10.1124/dmd.108.025619. Epub 2008 Dec 30. Drug Metab Dispos. 2009. PMID: 19116262 Free PMC article.
-
Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3.J Steroid Biochem Mol Biol. 2008 Dec;112(4-5):213-9. doi: 10.1016/j.jsbmb.2008.10.005. Epub 2008 Oct 21. J Steroid Biochem Mol Biol. 2008. PMID: 19000766 Free PMC article.
-
Novel activities of CYP11A1 and their potential physiological significance.J Steroid Biochem Mol Biol. 2015 Jul;151:25-37. doi: 10.1016/j.jsbmb.2014.11.010. Epub 2014 Nov 13. J Steroid Biochem Mol Biol. 2015. PMID: 25448732 Free PMC article. Review.
-
A new look at the most successful prodrugs for active vitamin D (D hormone): alfacalcidol and doxercalciferol.Molecules. 2009 Sep 29;14(10):3869-80. doi: 10.3390/molecules14103869. Molecules. 2009. PMID: 19924035 Free PMC article. Review.
Cited by
-
COVID-19 and Vitamin D: A lesson from the skin.Exp Dermatol. 2020 Sep;29(9):885-890. doi: 10.1111/exd.14170. Epub 2020 Sep 9. Exp Dermatol. 2020. PMID: 32779213 Free PMC article.
-
Differential expression of HPA axis homolog in the skin.Mol Cell Endocrinol. 2007 Feb;265-266:143-9. doi: 10.1016/j.mce.2006.12.012. Epub 2006 Dec 29. Mol Cell Endocrinol. 2007. PMID: 17197073 Free PMC article. Review.
-
Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives.Cell Biochem Biophys. 2020 Jun;78(2):165-180. doi: 10.1007/s12013-020-00913-6. Epub 2020 May 22. Cell Biochem Biophys. 2020. PMID: 32441029 Free PMC article. Review.
-
Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells.Drug Metab Dispos. 2011 Sep;39(9):1577-88. doi: 10.1124/dmd.111.040071. Epub 2011 Jun 15. Drug Metab Dispos. 2011. PMID: 21677063 Free PMC article.
-
Melanocyte receptors: clinical implications and therapeutic relevance.Dermatol Clin. 2007 Oct;25(4):541-57, viii-ix. doi: 10.1016/j.det.2007.06.005. Dermatol Clin. 2007. PMID: 17903613 Free PMC article. Review.
References
-
- Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. - PubMed
-
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. - PubMed
-
- Brown AJ, Ritter CS, Holliday LS, Knutson JC, Strugnell SA. Tissue distribution and activity studies of 1,24-dihydroxyvitamin D2, a metabolite of vitamin D2 with low calcemic activity in vivo. Biochem Pharmacol. 2004;68:1289–1296. - PubMed
-
- Albert DM, Kumar A, Strugnell SA, Darjatmoko SR, Lokken JM, Lindstrom MJ, Damico CM, Patel S. Effectiveness of 1alpha-hydroxyvitamin D2 in inhibiting tumor growth in a murine transgenic pigmented ocular tumor model. Arch Ophthalmol. 2004;122:1365–1369. - PubMed
-
- Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

