Clearance and prevention of prion infection in cell culture by anti-PrP antibodies
- PMID: 16817866
- PMCID: PMC1779824
- DOI: 10.1111/j.1460-9568.2006.04805.x
Clearance and prevention of prion infection in cell culture by anti-PrP antibodies
Abstract
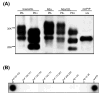
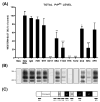
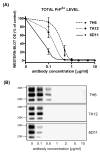
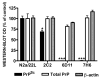
Prion diseases are transmissible and invariably fatal neurodegenerative disorders associated with a conformational transformation of the cellular prion protein (PrP(C)) into a self-replicating and proteinase K (PK)-resistant conformer, scrapie PrP (PrP(Sc)). Humoral immunity may significantly prolong the incubation period and even prevent disease in murine models of prionoses. However, the mechanism(s) of action of anti-PrP monoclonal antibodies (Mabs) remain(s) obscure. The murine neuroblastoma N2a cell line, infected with the 22L mouse-adapted scrapie strain, was used to screen a large library of Mabs with similar binding affinities to PrP, to identify those antibodies which could clear established infection and/or prevent infection de novo. Three Mabs were found capable of complete and persistent clearing of already-infected N2a cells of PrP(Sc). These antibodies were 6D11 (generated to PK-resistant PrP(Sc) and detecting PrP residues 93-109), and 7H6 and 7A12, which were raised against recombinant PrP and react with neighbouring epitopes of PrP residues 130-140 and 143-155, respectively. Mabs were found to interact with PrP(Sc) formation both on the cell surface and after internalization in the cytosol. Treatment with Mabs was not associated with toxicity nor did it result in decreased expression of PrP(C). Both preincubation of N2a cells with Mabs prior to exposure to 22L inoculum and preincubation of the inoculum with Mabs prior to infecting N2a cells resulted in a significant reduction in PrP(Sc) levels. Information provided in these studies is important for the rational design of humoral immune therapy for prion infection in animals and eventually in humans.
Figures




Similar articles
-
Anti-prion Protein Antibody 6D11 Restores Cellular Proteostasis of Prion Protein Through Disrupting Recycling Propagation of PrPSc and Targeting PrPSc for Lysosomal Degradation.Mol Neurobiol. 2019 Mar;56(3):2073-2091. doi: 10.1007/s12035-018-1208-4. Epub 2018 Jul 9. Mol Neurobiol. 2019. PMID: 29987703 Free PMC article.
-
Anti-PrP antibodies block PrPSc replication in prion-infected cell cultures by accelerating PrPC degradation.J Neurochem. 2004 Apr;89(2):454-63. doi: 10.1111/j.1471-4159.2004.02356.x. J Neurochem. 2004. PMID: 15056288 Free PMC article.
-
Human anti-prion antibodies block prion peptide fibril formation and neurotoxicity.J Biol Chem. 2012 Apr 13;287(16):12858-66. doi: 10.1074/jbc.M111.255836. Epub 2012 Feb 23. J Biol Chem. 2012. PMID: 22362783 Free PMC article.
-
Vaccine approaches to prevent and treat prion infection : progress and challenges.BioDrugs. 2008;22(1):45-52. doi: 10.2165/00063030-200822010-00005. BioDrugs. 2008. PMID: 18215090 Review.
-
Prion protein-specific antibodies for therapeutic intervention of transmissible spongiform encephalopathies.Expert Opin Biol Ther. 2006 Mar;6(3):293-300. doi: 10.1517/14712598.6.3.293. Expert Opin Biol Ther. 2006. PMID: 16503737 Review.
Cited by
-
Passive Immunization With a Novel Monoclonal Anti-PrP Antibody TW1 in an Alzheimer's Mouse Model With Tau Pathology.Front Aging Neurosci. 2021 Feb 25;13:640677. doi: 10.3389/fnagi.2021.640677. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33716717 Free PMC article.
-
Blocking the interaction between apolipoprotein E and Aβ reduces intraneuronal accumulation of Aβ and inhibits synaptic degeneration.Am J Pathol. 2013 May;182(5):1750-68. doi: 10.1016/j.ajpath.2013.01.034. Epub 2013 Mar 13. Am J Pathol. 2013. PMID: 23499462 Free PMC article.
-
Persistent propagation of variant Creutzfeldt-Jakob disease agent in murine spleen stromal cell culture with features of mesenchymal stem cells.J Virol. 2008 Nov;82(21):10959-62. doi: 10.1128/JVI.01085-08. Epub 2008 Aug 20. J Virol. 2008. PMID: 18715934 Free PMC article.
-
Anti-PrP Mab 6D11 suppresses PrP(Sc) replication in prion infected myeloid precursor line FDC-P1/22L and in the lymphoreticular system in vivo.Neurobiol Dis. 2009 May;34(2):267-78. doi: 10.1016/j.nbd.2009.01.013. Neurobiol Dis. 2009. PMID: 19385058 Free PMC article.
-
A novel, drug-based, cellular assay for the activity of neurotoxic mutants of the prion protein.J Biol Chem. 2010 Mar 5;285(10):7752-65. doi: 10.1074/jbc.M109.064949. Epub 2009 Nov 24. J Biol Chem. 2010. PMID: 19940127 Free PMC article.
References
-
- Adler V, Zeller B, Kryukov V, Kascsak R, Rubenstein R, Grossman A. Small, highly structured RNAs participate in the conversion of human recombinant Prp (Sen) to Prp (Res) in vitro . J Mol Biol. 2003;332:47–57. - PubMed
-
- Balter M. Spongiform disease. Experts downplay new vCJD fears. Science. 2002;289:1866–1867. - PubMed
-
- Carp RI, Meeker HC, Rubenstein R, Sigurdarson S, Papini M, Kascsak RJ, Kozlowski PB, Wisniewski HM. Characteristics of scrapie isolates derived from hay mites. J Neurovirol. 2000;6:137–144. - PubMed
-
- DeArmond SJ, Kretzschmar H, Prusiner SB. Prion diseases. In: Graham DI, Lantos P, editors. Greenfield’s Neuropathology. Arnold; London: 2002. pp. 273–324.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

