Testis and antler dysgenesis in sitka black-tailed deer on Kodiak Island, Alaska: Sequela of environmental endocrine disruption?
- PMID: 16818246
- PMCID: PMC1874179
- DOI: 10.1289/ehp.8052
Testis and antler dysgenesis in sitka black-tailed deer on Kodiak Island, Alaska: Sequela of environmental endocrine disruption?
Abstract
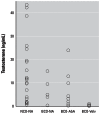
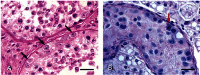
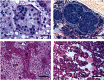
It had been observed that many male Sitka black-tailed deer (Odocoileus hemionus sitkensis) on Kodiak Island, Alaska, had abnormal antlers, were cryptorchid, and presented no evidence of hypospadias. We sought to better understand the problem and investigated 171 male deer for phenotypic aberrations and 12 for detailed testicular histopathology. For the low-lying Aliulik Peninsula (AP), 61 of 94 deer were bilateral cryptorchids (BCOs); 70% of these had abnormal antlers. Elsewhere on the Kodiak Archipelago, only 5 of 65 deer were BCOs. All 11 abdominal testes examined had no spermatogenesis but contained abnormalities including carcinoma in situ-like cells, possible precursors of seminoma; Sertoli cell, Leydig cell, and stromal cell tumors; carcinoma and adenoma of rete testis; and microlithiasis or calcifications. Cysts also were evident within the excurrent ducts. Two of 10 scrotal testes contained similar abnormalities, although spermatogenesis was ongoing. We cannot rule out that these abnormalities are linked sequelae of a mutation(s) in a founder animal, followed by transmission over many years and causing high prevalence only on the AP. However, based on lesions observed, we hypothesize that it is more likely that this testis-antler dysgenesis resulted from continuing exposure of pregnant females to an estrogenic environmental agent(s), thereby transforming testicular cells, affecting development of primordial antler pedicles, and blocking transabdominal descent of fetal testes. A browse (e.g., kelp) favored by deer in this locale might carry the putative estrogenic agent(s).
Figures



Similar articles
-
Testicular lesions and antler abnormalities in Colorado, USA mule deer (Odocoileus hemionus): a possible role for epizootic hemorrhagic disease virus.J Wildl Dis. 2015 Jan;51(1):166-76. doi: 10.7589/2014-03-067. J Wildl Dis. 2015. PMID: 25375947
-
Abnormal antler growth associated with testicular hypogonadism in red deer.J Wildl Dis. 1997 Jul;33(3):670-2. doi: 10.7589/0090-3558-33.3.670. J Wildl Dis. 1997. PMID: 9249723
-
Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection.Birth Defects Res A Clin Mol Teratol. 2010 Oct;88(10):910-9. doi: 10.1002/bdra.20707. Birth Defects Res A Clin Mol Teratol. 2010. PMID: 20865786 Review.
-
Reproductive dysgenesis in wildlife: a comparative view.Int J Androl. 2006 Feb;29(1):109-21. doi: 10.1111/j.1365-2605.2005.00631.x. Int J Androl. 2006. PMID: 16466531 Review.
-
Testicular dysgenesis syndrome: possible role of endocrine disrupters.Best Pract Res Clin Endocrinol Metab. 2006 Mar;20(1):77-90. doi: 10.1016/j.beem.2005.09.004. Best Pract Res Clin Endocrinol Metab. 2006. PMID: 16522521 Review.
Cited by
-
Effect of pre-fixation delay and freezing on mink testicular endpoints for environmental research.PLoS One. 2015 May 1;10(5):e0125139. doi: 10.1371/journal.pone.0125139. eCollection 2015. PLoS One. 2015. PMID: 25933113 Free PMC article.
-
Impact of environmental pollutants on the male: effects on germ cell differentiation.Anim Reprod Sci. 2008 Apr;105(1-2):144-57. doi: 10.1016/j.anireprosci.2007.11.020. Epub 2007 Nov 26. Anim Reprod Sci. 2008. PMID: 18155861 Free PMC article.
-
Possible fetal determinants of male infertility.Nat Rev Endocrinol. 2014 Sep;10(9):553-62. doi: 10.1038/nrendo.2014.97. Epub 2014 Jun 17. Nat Rev Endocrinol. 2014. PMID: 24935122 Review.
-
Cancer susceptibility and reproductive trade-offs: a model of the evolution of cancer defences.Philos Trans R Soc Lond B Biol Sci. 2015 Jul 19;370(1673):20140220. doi: 10.1098/rstb.2014.0220. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26056364 Free PMC article.
-
Introduction: The ecological relevance of chemically induced endocrine disruption in wildlife.Environ Health Perspect. 2006 Apr;114 Suppl 1(Suppl 1):7-8. doi: 10.1289/ehp.8046. Environ Health Perspect. 2006. PMID: 16818239 Free PMC article. No abstract available.
References
-
- Amann RP. 1990. Management of bulls to maximize sperm output. In: Proceedings of the Thirteenth Technical Conference, 20–21 April 1990, Milwaukee, WI. Columbia, MO: National Association of Animal Breeders, 84–91.
-
- Amann RP, Hammerstedt RH. In vitro evaluation of sperm quality: an opinion. J Androl. 1993;14:397–406. - PubMed
-
- Berndtson WE, Pickett BW, Nett TM. Reproductive physiology of the stallion. IV. Seasonal changes in the testosterone concentration of peripheral plasma. J Reprod Fertil. 1974;39:115–118. - PubMed
-
- Bornman MS, Barnhoorn IEJ, Dreyer L, Veeramachaneni DNR, De Jager C. 2004. Testicular degeneration coincident with fat residues of nonylphenol in the common eland (Tragelaphus oryx): a possible link to endocrine disruption [Abstract]? In: Proceedings of the CREDO Cluster Workshop on Ecological Relevance of Chemically-Induced Endocrine Disruption in Wildlife, 5–7 July 2004, Exeter, UK. Exeter, UK:University of Exeter, 59.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

