The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination
- PMID: 16818611
- PMCID: PMC1522077
- DOI: 10.1101/gad.375706
The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination
Abstract
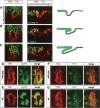
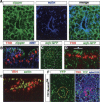
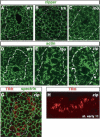
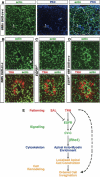
A major issue in morphogenesis is to understand how the activity of genes specifying cell fate affects cytoskeletal components that modify cell shape and induce cell movements. Here, we approach this question by investigating how a group of cells from an epithelial sheet initiate invagination to ultimately form the Drosophila tracheal tubes. We describe tracheal cell behavior at invagination and show that it is associated with, and requires, a distinct recruitment of Myosin II to the apical surface of cells at the invaginating edge. We show that this process is achieved by the activity of crossveinless-c, a gene coding for a RhoGAP and whose specific transcriptional activation in the tracheal cells is triggered by both the trachealess patterning gene and the EGF Receptor (EGFR) signaling pathway. Our results identify a developmental pathway linking cell fate genes and cell signaling pathways to intracellular modifications during tracheal cell invagination.
Figures



Similar articles
-
Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis.Development. 2006 Nov;133(21):4257-67. doi: 10.1242/dev.02588. Epub 2006 Oct 4. Development. 2006. PMID: 17021037
-
Repression of Wasp by JAK/STAT signalling inhibits medial actomyosin network assembly and apical cell constriction in intercalating epithelial cells.Development. 2009 Dec;136(24):4199-212. doi: 10.1242/dev.040402. Development. 2009. PMID: 19934015
-
Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila.Dev Cell. 2005 Dec;9(6):831-42. doi: 10.1016/j.devcel.2005.10.008. Dev Cell. 2005. PMID: 16326394
-
Compartmentalized morphogenesis in epithelia: from cell to tissue shape.Dev Dyn. 2005 Mar;232(3):685-94. doi: 10.1002/dvdy.20334. Dev Dyn. 2005. PMID: 15712202 Review.
-
Cell fate choices in Drosophila tracheal morphogenesis.Bioessays. 2000 Mar;22(3):219-26. doi: 10.1002/(SICI)1521-1878(200003)22:3<219::AID-BIES3>3.0.CO;2-A. Bioessays. 2000. PMID: 10684581 Review.
Cited by
-
Tissue remodelling through branching morphogenesis.Nat Rev Mol Cell Biol. 2009 Dec;10(12):831-42. doi: 10.1038/nrm2797. Nat Rev Mol Cell Biol. 2009. PMID: 19888266 Review.
-
Cell-Cell Contact and Receptor Tyrosine Kinase Signaling.Cold Spring Harb Perspect Biol. 2018 Jun 1;10(6):a029215. doi: 10.1101/cshperspect.a029215. Cold Spring Harb Perspect Biol. 2018. PMID: 28716887 Free PMC article. Review.
-
Systematic expression and loss-of-function analysis defines spatially restricted requirements for Drosophila RhoGEFs and RhoGAPs in leg morphogenesis.Mech Dev. 2011 Jan-Feb;128(1-2):5-17. doi: 10.1016/j.mod.2010.09.001. Epub 2010 Sep 17. Mech Dev. 2011. PMID: 20851182 Free PMC article.
-
DLC-1:a Rho GTPase-activating protein and tumour suppressor.J Cell Mol Med. 2007 Sep-Oct;11(5):1185-207. doi: 10.1111/j.1582-4934.2007.00098.x. J Cell Mol Med. 2007. PMID: 17979893 Free PMC article. Review.
-
Tube continued: morphogenesis of the Drosophila tracheal system.Curr Opin Cell Biol. 2010 Oct;22(5):633-9. doi: 10.1016/j.ceb.2010.07.016. Epub 2010 Aug 23. Curr Opin Cell Biol. 2010. PMID: 20739171 Free PMC article. Review.
References
-
- Barrio R., de Celis J.F., Bolshakov S., Kafatos F.C. Identification of regulatory regions driving the expression of the Drosophila spalt complex at different developmental stages. Dev. Biol. 1999;215:33–47. - PubMed
-
- Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta. 2003;1603:47–82. - PubMed
-
- Bloor J.W., Kiehart D.P. zipper Nonmuscle myosin-II functions downstream of PS2 integrin in Drosophila myogenesis and is necessary for myofibril formation. Dev. Biol. 2001;239:215–228. - PubMed
-
- Boube M., Llimargas M., Casanova J. Cross-regulatory interactions among tracheal genes support a co-operative model for the induction of tracheal fates in the Drosophila embryo. Mech. Dev. 2000;91:271–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
