Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells
- PMID: 16818676
- PMCID: PMC2118333
- DOI: 10.1084/jem.20060468
Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells
Abstract
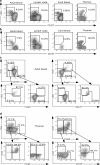
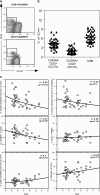
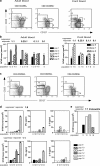
Abnormalities in CD4(+)CD25(+)Foxp3(+) regulatory T (T reg) cells have been implicated in susceptibility to allergic, autoimmune, and immunoinflammatory conditions. However, phenotypic and functional assessment of human T reg cells has been hampered by difficulty in distinguishing between CD25-expressing activated and regulatory T cells. Here, we show that expression of CD127, the alpha chain of the interleukin-7 receptor, allows an unambiguous flow cytometry-based distinction to be made between CD127(lo) T reg cells and CD127(hi) conventional T cells within the CD25(+)CD45RO(+)RA(-) effector/memory and CD45RA(+)RO(-) naive compartments in peripheral blood and lymph node. In healthy volunteers, peripheral blood CD25(+)CD127(lo) cells comprised 6.35 +/- 0.26% of CD4(+) T cells, of which 2.05 +/- 0.14% expressed the naive subset marker CD45RA. Expression of FoxP3 protein and the CD127(lo) phenotype were highly correlated within the CD4(+)CD25(+) population. Moreover, both effector/memory and naive CD25(+)CD127(lo) cells manifested suppressive activity in vitro, whereas CD25(+)CD127(hi) cells did not. Cell surface expression of CD127 therefore allows accurate estimation of T reg cell numbers and isolation of pure populations for in vitro studies and should contribute to our understanding of regulatory abnormalities in immunopathic diseases.
Figures


References
-
- Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562. - PubMed
-
- Crispin, J.C., A. Martinez, and J. Alcocer-Varela. 2003. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J. Autoimmun. 21:273–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

