Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release
- PMID: 16818724
- PMCID: PMC2064170
- DOI: 10.1083/jcb.200511054
Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release
Abstract
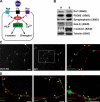
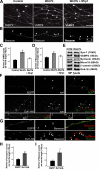
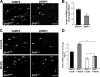
Proper dialogue between presynaptic neurons and their targets is essential for correct synaptic assembly and function. At central synapses, Wnt proteins function as retrograde signals to regulate axon remodeling and the accumulation of presynaptic proteins. Loss of Wnt7a function leads to defects in the localization of presynaptic markers and in the morphology of the presynaptic axons. We show that loss of function of Dishevelled-1 (Dvl1) mimics and enhances the Wnt7a phenotype in the cerebellum. Although active zones appear normal, electrophysiological recordings in cerebellar slices from Wnt7a/Dvl1 double mutant mice reveal a defect in neurotransmitter release at mossy fiber-granule cell synapses. Deficiency in Dvl1 decreases, whereas exposure to Wnt increases, synaptic vesicle recycling in mossy fibers. Dvl increases the number of Bassoon clusters, and like other components of the Wnt pathway, it localizes to synaptic sites. These findings demonstrate that Wnts signal across the synapse on Dvl-expressing presynaptic terminals to regulate synaptic assembly and suggest a potential novel function for Wnts in neurotransmitter release.
Figures




References
-
- Cadigan, K.M., and Y.I. Liu. 2006. Wnt signaling: complexity at the surface. J. Cell Sci. 119:395–402. - PubMed
-
- Christopherson, K.S., E.M. Ullian, C.C. Stokes, C.E. Mullowney, J.W. Hell, A. Agah, J. Lawler, D.F. Mosher, P. Bornstein, and B.A. Barres. 2005. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 120:421–433. - PubMed
-
- Ciani, L., and P.C. Salinas. 2005. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 6:351–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

