Antigen-experienced T cells limit the priming of naive T cells during infection with Leishmania major
- PMID: 16818747
- PMCID: PMC2696341
- DOI: 10.4049/jimmunol.177.2.925
Antigen-experienced T cells limit the priming of naive T cells during infection with Leishmania major
Abstract
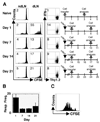
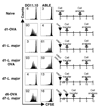
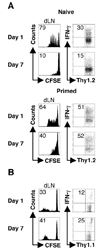
One mechanism to control immune responses following infection is to rapidly down-regulate Ag presentation, which has been observed in acute viral and bacterial infections. In this study, we describe experiments designed to address whether Ag presentation is decreased after an initial response to Leishmania major. Naive alphabeta-Leishmania-specific (ABLE) TCR transgenic T cells were adoptively transferred into mice at various times after L. major infection to determine the duration of presentation of parasite-derived Ags. ABLE T cells responded vigorously at the initiation of infection, but the ability to prime these cells quickly diminished, independent of IL-10, regulatory T cells, or Ag load. However, Ag-experienced clonal and polyclonal T cell populations could respond, indicating that the diminution in naive ABLE cell responses was not due to lack of Ag presentation. Because naive T cell priming could be restored by removal of the endogenous T cell population, or adoptive transfer of Ag-pulsed dendritic cells, it appears that T cells that have previously encountered Ag during infection compete with naive Ag-specific T cells. These results suggest that during L. major infection Ag-experienced T cells, rather than naive T cells, may be primarily responsible for sustaining the immune response.
Figures






Similar articles
-
Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection.J Immunol. 2006 Jul 15;177(2):1250-6. doi: 10.4049/jimmunol.177.2.1250. J Immunol. 2006. PMID: 16818784
-
Inefficient cross-presentation limits the CD8+ T cell response to a subdominant tumor antigen epitope.J Immunol. 2005 Jul 15;175(2):700-12. doi: 10.4049/jimmunol.175.2.700. J Immunol. 2005. PMID: 16002665
-
STAT1 expression in dendritic cells, but not T cells, is required for immunity to Leishmania major.J Immunol. 2007 Jun 1;178(11):7259-66. doi: 10.4049/jimmunol.178.11.7259. J Immunol. 2007. PMID: 17513775
-
Priming by microbial antigens from the intestinal flora determines the ability of CD4+ T cells to rapidly secrete IL-4 in BALB/c mice infected with Leishmania major.J Immunol. 2000 Nov 15;165(10):5637-45. doi: 10.4049/jimmunol.165.10.5637. J Immunol. 2000. PMID: 11067920
-
Experimental cutaneous leishmaniasis: induction and regulation of T cells following infection of mice with Leishmania major.Chem Immunol. 1998;70:60-80. doi: 10.1159/000058698. Chem Immunol. 1998. PMID: 9509670 Review. No abstract available.
Cited by
-
Tentative T cells: memory cells are quick to respond, but slow to divide.PLoS Pathog. 2008 Apr 11;4(4):e1000041. doi: 10.1371/journal.ppat.1000041. PLoS Pathog. 2008. PMID: 18404208 Free PMC article.
-
CD4 T cells in protection from influenza virus: Viral antigen specificity and functional potential.Immunol Rev. 2018 Jul;284(1):91-105. doi: 10.1111/imr.12662. Immunol Rev. 2018. PMID: 29944766 Free PMC article. Review.
-
Tracking antigen-specific CD4+ T cells throughout the course of chronic Leishmania major infection in resistant mice.Eur J Immunol. 2013 Feb;43(2):427-38. doi: 10.1002/eji.201242715. Epub 2012 Dec 11. Eur J Immunol. 2013. PMID: 23109292 Free PMC article.
-
Regulation of CD4+ T-cell contraction during pathogen challenge.Immunol Rev. 2010 Jul;236:110-24. doi: 10.1111/j.1600-065X.2010.00921.x. Immunol Rev. 2010. PMID: 20636812 Free PMC article. Review.
-
The early generation of a heterogeneous CD4+ T cell response to Leishmania major.J Immunol. 2010 Aug 15;185(4):2416-23. doi: 10.4049/jimmunol.1000483. Epub 2010 Jul 12. J Immunol. 2010. PMID: 20624946 Free PMC article.
References
-
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. - PubMed
-
- Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10:1104–1110. - PubMed
-
- Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. - PubMed
-
- van Faassen H, Dudani R, Krishnan L, Sad S. Prolonged antigen presentation, APC−, and CD8+ T cell turnover during mycobacterial infection: comparison with Listeria monocytogenes. J Immunol. 2004;172:3491–3500. - PubMed
-
- van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174:5341–5350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

