Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin
- PMID: 16818890
- PMCID: PMC1502310
- DOI: 10.1073/pnas.0602493103
Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin
Abstract
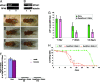
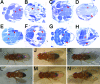
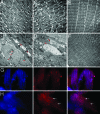
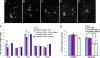
Mutations in Pink1, a gene encoding a Ser/Thr kinase with a mitochondrial-targeting signal, are associated with Parkinson's disease (PD), the most common movement disorder characterized by selective loss of dopaminergic neurons. The mechanism by which loss of Pink1 leads to neurodegeneration is not understood. Here we show that inhibition of Drosophila Pink1 (dPink1) function results in energy depletion, shortened lifespan, and degeneration of select indirect flight muscles and dopaminergic neurons. The muscle pathology was preceded by mitochondrial enlargement and disintegration. These phenotypes could be rescued by the wild type but not the pathogenic C-terminal deleted form of human Pink1 (hPink1). The muscle and dopaminergic phenotypes associated with dPink1 inactivation show similarity to that seen in parkin mutant flies and could be suppressed by the overexpression of Parkin but not DJ-1. Consistent with the genetic rescue results, we find that, in dPink1 RNA interference (RNAi) animals, the level of Parkin protein is significantly reduced. Together, these results implicate Pink1 and Parkin in a common pathway that regulates mitochondrial physiology and cell survival in Drosophila.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Dunnett S. B., Bjorklund A. Nature. 1999;399:A32–A39. - PubMed
-
- Dawson T. M., Dawson V. L. Science. 2003;302:819–822. - PubMed
-
- Bertoli-Avella A. M., Oostra B. A., Heutink P. Hum. Genet. 2004;114:413–438. - PubMed
-
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., et al. Science. 1997;276:2045–2047. - PubMed
-
- Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Nature. 1998;392:605–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

