Parathyroid hormone for the treatment of osteoporosis: a systematic review
- PMID: 16818910
- PMCID: PMC1482742
- DOI: 10.1503/cmaj.050929
Parathyroid hormone for the treatment of osteoporosis: a systematic review
Abstract
Background: Human parathyroid hormone (hPTH)(1-34) was approved in 2004 for the treatment of severe osteoporosis. Members of the Osteoporosis Canada clinical guidelines committee conducted a systematic review of randomized controlled trials (RCTs) to assess the efficacy and safety of hPTH for fracture prevention in postmenopausal women and men with osteoporosis.
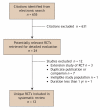
Methods: We searched MEDLINE, EMBASE, HTA, Current Contents and the Cochrane Controlled Trials Registry for published data from 1966 to February 2005. A systematic literature search for RCTs was conducted using the Cochrane Collaborative approach. We identified 12 trials that randomly assigned patients either to hPTH or placebo or to hPTH or an active comparator and were at least 1 year in duration. Outcomes included change in bone mineral density (BMD), fractures, back pain and adverse events. Two independent reviewers abstracted data on study characteristics and outcomes.
Results: hPTH(1-34) significantly increases lumbar spine BMD, with smaller increases at the femoral neck and total hip. hPTH(1-84) significantly increases lumbar spine BMD. The data show a significant reduction in both vertebral and nonvertebral fractures with hPTH(1-34) in postmenopausal women with previous vertebral fractures. There were no data on fractures comparing the approved dose of hPTH(1-34) with active comparators.
Interpretation: There is Level I evidence that hPTH(1-34) significantly increases BMD at all skeletal sites except the radius and significantly reduces the risk of new vertebral and nonvertebral fractures in postmenopausal women with prior fractures.
Figures
Comment in
-
Clinical practice guidelines for the use of parathyroid hormone in the treatment of osteoporosis.CMAJ. 2006 Jul 4;175(1):48. doi: 10.1503/cmaj.060624. CMAJ. 2006. PMID: 16818909 Free PMC article. No abstract available.
-
Review: human parathyroid hormone reduces fractures and increases bone mineral density in severe osteoporosis.ACP J Club. 2006 Nov-Dec;145(3):71. ACP J Club. 2006. PMID: 17080983 No abstract available.
References
-
- Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 2005;20:177-84. - PubMed
-
- Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 2005;26:688-703. - PubMed
-
- Murray TM, Rao LG, Diveti P, et al. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr Rev 2005;26:78-113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical