Enterobactin protonation and iron release: structural characterization of the salicylate coordination shift in ferric enterobactin
- PMID: 16819888
- PMCID: PMC3188320
- DOI: 10.1021/ja062046j
Enterobactin protonation and iron release: structural characterization of the salicylate coordination shift in ferric enterobactin
Abstract
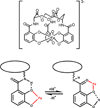
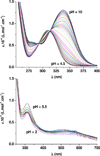
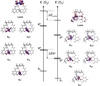
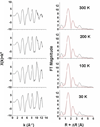
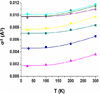
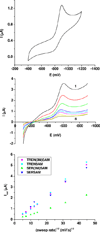
The siderophore enterobactin (Ent) is produced by many species of enteric bacteria to mediate iron uptake. This iron scavenger can be reincorporated by the bacteria as the ferric complex [Fe(III)(Ent)](3)(-) and is subsequently hydrolyzed by an esterase to facilitate intracellular iron release. Recent literature reports on altered protein recognition and binding of modified enterobactin increase the significance of understanding the structural features and solution chemistry of ferric enterobactin. The structure of the neutral protonated ferric enterobactin complex [Fe(III)(H(3)Ent)](0) has been the source of some controversy and confusion in the literature. To demonstrate the proposed change of coordination from the tris-catecholate [Fe(III)(Ent)](3)(-) to the tris-salicylate [Fe(III)(H(3)Ent)](0) upon protonation, the coordination chemistry of two new model compounds N,N',N''-tris[2-(hydroxybenzoyl)carbonyl]cyclotriseryl trilactone (SERSAM) and N,N',N''-tris[2-hydroxy,3-methoxy(benzoyl)carbonyl]cyclotriseryl trilactone (SER(3M)SAM) was examined in solution and solid state. Both SERSAM and SER(3M)SAM form tris-salicylate ferric complexes with spectroscopic and solution thermodynamic properties (with log beta(110)() values of 39 and 38 respectively) similar to those of [Fe(III)(H(3)Ent)](0). The fits of EXAFS spectra of the model ferric complexes and the two forms of ferric enterobactin provided bond distances and disorder factors in the metal coordination sphere for both coordination modes. The protonated [Fe(III)(H(3)Ent)](0) complex (d(Fe)(-)(O) = 1.98 A, sigma(2)(stat)(O) = 0.00351(10) A(2)) exhibits a shorter average Fe-O bond length but a much higher static Debye-Waller factor for the first oxygen shell than the catecholate [Fe(III)(Ent)](3)(-) complex (d(Fe)(-)(O) = 2.00 A, sigma(2)(stat)(O) = 0.00067(14) A(2)). (1)H NMR spectroscopy was used to monitor the amide bond rotation between the catecholate and salicylate geometries using the gallic complexes of enterobactin: [Ga(III)(Ent)](3)(-) and [Ga(III)(H(3)Ent)](0). The ferric salicylate complexes display quasi-reversible reduction potentials from -89 to -551 mV (relative to the normal hydrogen electrode NHE) which supports the feasibility of a low pH iron release mechanism facilitated by biological reductants.
Figures



Similar articles
-
Catecholate/salicylate heteropodands: demonstration of a catecholate to salicylate coordination change.Inorg Chem. 2000 Aug, 7;39(16):3624-31. doi: 10.1021/ic990608c. Inorg Chem. 2000. PMID: 11196825
-
Fe(III) coordination properties of two new saccharide-based enterobactin analogues: methyl 2,3,4-tris-O-[N-[2,3-di(hydroxy)benzoyl-glycyl]-aminopropyl]-alpha-D-glucopyranoside and methyl 2,3,4-tris-O-[N-[2,3-di-(hydroxy)-benzoyl]-aminopropyl]-alpha-D-glucopyranoside.Inorg Chem. 2001 Dec 31;40(27):7079-86. doi: 10.1021/ic0104003. Inorg Chem. 2001. PMID: 11754294
-
The siderocalin/enterobactin interaction: a link between mammalian immunity and bacterial iron transport.J Am Chem Soc. 2008 Aug 27;130(34):11524-34. doi: 10.1021/ja803524w. Epub 2008 Aug 5. J Am Chem Soc. 2008. PMID: 18680288 Free PMC article.
-
Enterobactin: an archetype for microbial iron transport.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3584-8. doi: 10.1073/pnas.0630018100. Epub 2003 Mar 24. Proc Natl Acad Sci U S A. 2003. PMID: 12655062 Free PMC article. Review.
-
The chemical biology and coordination chemistry of putrebactin, avaroferrin, bisucaberin, and alcaligin.J Biol Inorg Chem. 2018 Oct;23(7):969-982. doi: 10.1007/s00775-018-1585-1. Epub 2018 Jun 26. J Biol Inorg Chem. 2018. PMID: 29946977 Review.
Cited by
-
Human Urinary Composition Controls Antibacterial Activity of Siderocalin.J Biol Chem. 2015 Jun 26;290(26):15949-60. doi: 10.1074/jbc.M115.645812. Epub 2015 Apr 10. J Biol Chem. 2015. PMID: 25861985 Free PMC article.
-
Structure and dynamics of Type III periplasmic proteins VcFhuD and VcHutB reveal molecular basis of their distinctive ligand binding properties.Sci Rep. 2017 Feb 20;7:42812. doi: 10.1038/srep42812. Sci Rep. 2017. PMID: 28216648 Free PMC article.
-
Human Metabolome-derived Cofactors Are Required for the Antibacterial Activity of Siderocalin in Urine.J Biol Chem. 2016 Dec 9;291(50):25901-25910. doi: 10.1074/jbc.M116.759183. Epub 2016 Oct 25. J Biol Chem. 2016. PMID: 27780864 Free PMC article.
-
New Insights into Aspirin's Anticancer Activity: The Predominant Role of Its Iron-Chelating Antioxidant Metabolites.Antioxidants (Basel). 2024 Dec 29;14(1):29. doi: 10.3390/antiox14010029. Antioxidants (Basel). 2024. PMID: 39857363 Free PMC article. Review.
-
Determination of the Molecular Structures of Ferric Enterobactin and Ferric Enantioenterobactin Using Racemic Crystallography.J Am Chem Soc. 2017 Oct 25;139(42):15245-15250. doi: 10.1021/jacs.7b09375. Epub 2017 Oct 17. J Am Chem Soc. 2017. PMID: 28956921 Free PMC article.
References
-
-
Coordination Chemistry of Microbial Iron Transport. 75. Part 74: Dertz EA, Raymond KN. Inorg. Chem. 2006 In Press.
-
-
- Earhart CF. In: Iron Transport in Bacteria: Molecular Genetics, Biochemistry, Microbial Pathogenesis and Ecology. Crosa JH, Payne SM, editors. Washington, D.C.: ASM Press; 2004. pp. 133–146.
-
- Walsh CT, Marshall CG. In: Iron Transport in Bacteria: Molecular Genetics, Biochemistry, Microbial Pathogenesis and Ecology. Crosa JH, Payne SM, editors. Washington, D.C.: ASM Press; 2004. pp. 18–37.
-
- Boukhalfa H, Crumbliss AL. BioMetals. 2002;15:325–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

