Increased protein glycation in fructosamine 3-kinase-deficient mice
- PMID: 16819943
- PMCID: PMC1609913
- DOI: 10.1042/BJ20060684
Increased protein glycation in fructosamine 3-kinase-deficient mice
Abstract
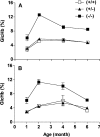
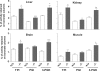
Amines, including those present on proteins, spontaneously react with glucose to form fructosamines in a reaction known as glycation. In the present paper, we have explored, through a targeted gene inactivation approach, the role of FN3K (fructosamine 3-kinase), an intracellular enzyme that phosphorylates free and protein-bound fructose-epsilon-lysines and which is potentially involved in protein repair. Fn3k-/- mice looked healthy and had normal blood glucose and serum fructosamine levels. However, their level of haemoglobin-bound fructosamines was approx. 2.5-fold higher than that of control (Fn3k+/+) or Fn3k+/- mice. Other intracellular proteins were also significantly more glycated in Fn3k-/- mice in erythrocytes (1.8-2.2-fold) and in brain, kidney, liver and skeletal muscle (1.2-1.8-fold), indicating that FN3K removes fructosamines from intracellular proteins in vivo. The urinary excretion of free fructose-epsilon-lysine was 10-20-fold higher in fed mice compared with mice starved for 36 h, and did not differ between fed Fn3k+/+ and Fn3k-/- mice, indicating that food is the main source of urinary fructose-epsilon-lysine in these mice and that FN3K does not participate in the metabolism of food-derived fructose-epsilon-lysine. However, in starved animals, the urinary excretion of fructose-epsilon-lysine was 2.5-fold higher in Fn3k-/- mice compared with Fn3k+/+ or Fn3k+/- mice. Furthermore, a marked increase (5-13-fold) was observed in the concentration of free fructose-epsilon-lysine in tissues of fed Fn3k-/- mice compared with control mice, indicating that FN3K participates in the metabolism of endogenously produced fructose-epsilon-lysine. Taken together, these data indicate that FN3K serves as a protein repair enzyme and also in the metabolism of endogenously produced free fructose-epsilon-lysine.
Figures



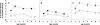
Comment in
-
The fructosamine 3-kinase knockout mouse: a tool for testing the glycation hypothesis of intracellular protein damage in diabetes and aging.Biochem J. 2006 Oct 15;399(2):e11-3. doi: 10.1042/BJ20061232. Biochem J. 2006. PMID: 16987105 Free PMC article.
Similar articles
-
Effects of fructosamine-3-kinase deficiency on function and survival of mouse pancreatic islets after prolonged culture in high glucose or ribose concentrations.Am J Physiol Endocrinol Metab. 2010 Mar;298(3):E586-96. doi: 10.1152/ajpendo.00503.2009. Epub 2009 Dec 15. Am J Physiol Endocrinol Metab. 2010. PMID: 20009024
-
Enzymatic repair of Amadori products.Amino Acids. 2012 Apr;42(4):1143-50. doi: 10.1007/s00726-010-0780-3. Epub 2010 Oct 22. Amino Acids. 2012. PMID: 20967558 Review.
-
Plant ribulosamine/erythrulosamine 3-kinase, a putative protein-repair enzyme.Biochem J. 2005 Jun 15;388(Pt 3):795-802. doi: 10.1042/BJ20041976. Biochem J. 2005. PMID: 15705060 Free PMC article.
-
Intrinsic toxicity of glucose, due to non-enzymatic glycation, is controlled in-vivo by deglycation systems including: FN3K-mediated deglycation of fructosamines and transglycation of aldosamines.Med Hypotheses. 2005;65(2):337-48. doi: 10.1016/j.mehy.2005.02.017. Med Hypotheses. 2005. PMID: 15922110
-
Enzymatic deglycation--a new paradigm or an epiphenomenon?Biochem Soc Trans. 2003 Dec;31(Pt 6):1428-32. doi: 10.1042/bst0311428. Biochem Soc Trans. 2003. PMID: 14641081 Review.
Cited by
-
The fructosamine 3-kinase knockout mouse: a tool for testing the glycation hypothesis of intracellular protein damage in diabetes and aging.Biochem J. 2006 Oct 15;399(2):e11-3. doi: 10.1042/BJ20061232. Biochem J. 2006. PMID: 16987105 Free PMC article.
-
Anti-Glucotoxicity Effect of Phytoconstituents via Inhibiting MGO-AGEs Formation and Breaking MGO-AGEs.Int J Mol Sci. 2023 Apr 21;24(8):7672. doi: 10.3390/ijms24087672. Int J Mol Sci. 2023. PMID: 37108833 Free PMC article. Review.
-
Advanced glycation end products, diabetes and ageing.Z Gerontol Geriatr. 2007 Oct;40(5):349-56. doi: 10.1007/s00391-007-0484-9. Z Gerontol Geriatr. 2007. PMID: 17943238 Review.
-
Role of fructosamine-3-kinase in protecting against the onset of microvascular and macrovascular complications in patients with T2DM.BMJ Open Diabetes Res Care. 2020 May;8(1):e001256. doi: 10.1136/bmjdrc-2020-001256. BMJ Open Diabetes Res Care. 2020. PMID: 32467223 Free PMC article.
-
Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects.Oxid Med Cell Longev. 2020 Mar 18;2020:3818196. doi: 10.1155/2020/3818196. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32256950 Free PMC article. Review.
References
-
- Hodge J. E. The Amadori rearrangement. Adv. Carbohydr. Chem. 1955;10:169–205. - PubMed
-
- Baynes J. W., Watkins N. G., Fisher C. I., Hull C. J., Patrick J. S., Ahmed M. U., Dunn J. A., Thorpe S. R. The Amadori product on protein: structure and reactions. Prog. Clin. Biol. Res. 1989;304:43–67. - PubMed
-
- Brownlee M. Glycation and diabetic complications. Diabetes. 1994;43:836–841. - PubMed
-
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann. Intern. Med. 1984;101:527–537. - PubMed
-
- Delpierre G., Rider M. H., Collard F., Stroobant V., Vanstapel F., Santos H., Van Schaftingen E. Identification, cloning, and heterologous expression of a mammalian fructosamine-3-kinase. Diabetes. 2000;49:1627–1634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

