Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics
- PMID: 16820043
- PMCID: PMC3226109
- DOI: 10.1186/ar1909
Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics
Abstract
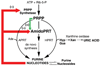
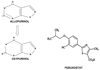
Although dietary, genetic, or disease-related excesses in urate production may contribute to hyperuricemia, impaired renal excretion of uric acid is the dominant cause of hyperuricemia in the majority of patients with gout. The aims of this review are to highlight exciting and clinically pertinent advances in our understanding of how uric acid is reabsorbed by the kidney under the regulation of urate transporter (URAT)1 and other recently identified urate transporters; to discuss urate-lowering agents in clinical development; and to summarize the limitations of currently available antihyperuricemic drugs. The use of uricosuric drugs to treat hyperuricemia in patients with gout is limited by prior urolothiasis or renal dysfunction. For this reason, our discussion focuses on the development of the novel xanthine oxidase inhibitor febuxostat and modified recombinant uricase preparations.
Figures

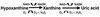



References
-
- Becker MA, Jolly M. In: Arthritis and Allied Conditions. 15. Koopman WJ, Moreland LW, editor. Philadelphia: Lippincott, Williams & Wilkins; 2005. Clinical gout and the pathogenesis of hyperuricemia; pp. 2303–2339.
-
- Wyngaarden JB, Kelley WN. Gout and Hyperuricemia. New York: Grune & Stratton; 1976. pp. 1–512.
-
- Benedict JD, Forsham PH, Stetten DeW Jr. The metabolism of uric acid in the normal and gouty human studied with the aid of isotopic uric acid. J Biol Chem. 1949;181:183–193. - PubMed
-
- Sorensen LB. Degradation of uric acid in man. Metabolism. 1959;8:687–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

