Old yellow enzymes protect against acrolein toxicity in the yeast Saccharomyces cerevisiae
- PMID: 16820484
- PMCID: PMC1489299
- DOI: 10.1128/AEM.00526-06
Old yellow enzymes protect against acrolein toxicity in the yeast Saccharomyces cerevisiae
Abstract
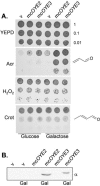
Acrolein is a ubiquitous reactive aldehyde which is formed as a product of lipid peroxidation in biological systems. In this present study, we screened the complete set of viable deletion strains in Saccharomyces cerevisiae for sensitivity to acrolein to identify cell functions involved in resistance to reactive aldehydes. We identified 128 mutants whose gene products are localized throughout the cell. Acrolein-sensitive mutants were distributed among most major biological processes but particularly affected gene expression, metabolism, and cellular signaling. Surprisingly, the screen did not identify any antioxidants or similar stress-protective molecules, indicating that acrolein toxicity may not be mediated via reactive oxygen species. Most strikingly, a mutant lacking an old yellow enzyme (OYE2) was identified as being acrolein sensitive. Old yellow enzymes are known to reduce alpha,beta-unsaturated carbonyl compounds in vitro, but their physiological roles have remained uncertain. We show that mutants lacking OYE2, but not OYE3, are sensitive to acrolein, and overexpression of both isoenzymes increases acrolein tolerance. Our data indicate that OYE2 is required for basal levels of tolerance, whereas OYE3 expression is particularly induced following acrolein stress. Despite the range of alpha,beta-unsaturated carbonyl compounds that have been identified as substrates of old yellow enzymes in vitro, we show that old yellow enzymes specifically mediate resistance to small alpha,beta-unsaturated carbonyl compounds, such as acrolein, in vivo.
Figures



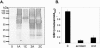

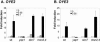
References
-
- Debouzy, J.-C., F. Fauvelle, H. Vezin, B. Brasme, and Y. Chancerelle. 1992. Interaction of the malondialdehyde molecule with membranes. Biochem. Pharmacol. 44:1787-1793. - PubMed
-
- Esterbauer, H. 1993. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 57(Suppl.):779S-786S. - PubMed
-
- Esterbauer, H., R. J. Schaur, and H. Zollner. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free Radic. Biol. Med. 11:81-128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

