Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil
- PMID: 16820495
- PMCID: PMC1489341
- DOI: 10.1128/AEM.03076-05
Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil
Abstract
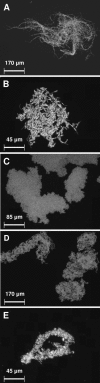
Bacillus is commonly isolated from soils, with organisms of Bacillus cereus sensu lato being prevalent. Knowledge of the ecology of B. cereus and other Bacillus species in soil is far from complete. While the older literature favors a model of growth on soil-associated organic matter, the current paradigm is that B. cereus sensu lato germinates and grows in association with animals or plants, resulting in either symbiotic or pathogenic interactions. An in terra approach to study soil-associated bacteria is described, using filter-sterilized soil-extracted soluble organic matter (SESOM) and artificial soil microcosms (ASM) saturated with SESOM. B. cereus ATCC 14579 displayed a life cycle, with the ability to germinate, grow, and subsequently sporulate in both the liquid SESOM extract and in ASM inserted into wells in agar medium. Cells grew in liquid SESOM without separating, forming multicellular structures that coalesced to form clumps and encasing the ensuing spores in an extracellular matrix. Bacillus was able to translocate from the point of inoculation through soil microcosms as shown by the emergence of outgrowths on the surrounding agar surface. Microscopic inspection revealed bundles of parallel chains inside the soil. The motility inhibitor L-ethionine failed to suppress outgrowth, ruling out translocation by a flagellar-mediated mechanism such as swimming or swarming. Bacillus subtilis subsp. subtilis Marburg and four Bacillus isolates taken at random from soils also displayed a life cycle in SESOM and ASM and were all able to translocate through ASM, even in presence of L-ethionine. These data indicate that B. cereus is a saprophytic bacterium that is able to grow in soil and furthermore that it is adapted to translocate by employing a multicellular mode of growth.
Figures




References
-
- Andreev, J., P. A. Dibrov, D. Klein, and S. Braun. 1994. Chemotaxis, sporulation, and larvicide production in Bacillus sphaericus 2362. The influence of l-ethionine, and of aminophenylboronic acid. FEBS Lett. 349:416-419. - PubMed
-
- Chattopadhyay, A., N. B. Bhatnagar, and R. Bhatnagar. 2004. Bacterial insecticidal toxins. Crit. Rev. Microbiol. 30:33-54. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

