Use of laccase as a novel, versatile reporter system in filamentous fungi
- PMID: 16820501
- PMCID: PMC1489370
- DOI: 10.1128/AEM.00060-06
Use of laccase as a novel, versatile reporter system in filamentous fungi
Abstract
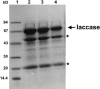
Laccases are copper-containing enzymes which oxidize phenolic substrates and transfer the electrons to oxygen. Many filamentous fungi contain several laccase-encoding genes, but their biological roles are mostly not well understood. The main interest in laccases in biotechnology is their potential to be used to detoxify phenolic substances. We report here on a novel application of laccases as a reporter system in fungi. We purified a laccase enzyme from the ligno-cellulolytic ascomycete Stachybotrys chartarum. It oxidized the artificial substrate 2,2'-azino-di-(3-ethylbenzthiazolinsulfonate) (ABTS). The corresponding gene was isolated and expressed in Aspergillus nidulans, Aspergillus niger, and Trichoderma reesei. Heterologously expressed laccase activity was monitored in colorimetric enzyme assays and on agar plates with ABTS as a substrate. The use of laccase as a reporter was shown in a genetic screen for the isolation of improved T. reesei cellulase production strains. In addition to the laccase from S. charatarum, we tested the application of three laccases from A. nidulans (LccB, LccC, and LccD) as reporters. Whereas LccC oxidized ABTS (Km = 0.3 mM), LccD did not react with ABTS but with DMA/ADBP (3,5-dimethylaniline/4-amino-2,6-dibromophenol). LccB reacted with DMA/ADBP and showed weak activity with ABTS. The different catalytic properties of LccC and LccD allow simultaneous use of these two laccases as reporters in one fungal strain.
Figures




Similar articles
-
The Aspergillus niger multicopper oxidase family: analysis and overexpression of laccase-like encoding genes.Microb Cell Fact. 2011 Oct 8;10:78. doi: 10.1186/1475-2859-10-78. Microb Cell Fact. 2011. PMID: 21981827 Free PMC article.
-
Heterologous laccase production and its role in industrial applications.Bioeng Bugs. 2010 Jul-Aug;1(4):252-62. doi: 10.4161/bbug.1.4.11438. Bioeng Bugs. 2010. PMID: 21327057 Free PMC article. Review.
-
[Mechanisms and regulation of enzymatic hydrolysis of cellulose in filamentous fungi: classical cases and new models].Rev Iberoam Micol. 2015 Jan-Mar;32(1):1-12. doi: 10.1016/j.riam.2013.10.009. Epub 2014 Mar 7. Rev Iberoam Micol. 2015. PMID: 24607657 Review. Spanish.
-
Improved biomass saccharification by Trichoderma reesei through heterologous expression of lacA gene from Trametes sp. AH28-2.J Biosci Bioeng. 2012 Jun;113(6):697-703. doi: 10.1016/j.jbiosc.2012.01.016. Epub 2012 Mar 3. J Biosci Bioeng. 2012. PMID: 22387233
-
Immobilization of LccC Laccase from Aspergillus nidulans on Hard Surfaces via Fungal Hydrophobins.Appl Environ Microbiol. 2016 Oct 14;82(21):6395-6402. doi: 10.1128/AEM.01413-16. Print 2016 Nov 1. Appl Environ Microbiol. 2016. PMID: 27565614 Free PMC article.
Cited by
-
Stachybotrys chartarum-A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance.J Fungi (Basel). 2022 May 12;8(5):504. doi: 10.3390/jof8050504. J Fungi (Basel). 2022. PMID: 35628759 Free PMC article. Review.
-
The Aspergillus niger multicopper oxidase family: analysis and overexpression of laccase-like encoding genes.Microb Cell Fact. 2011 Oct 8;10:78. doi: 10.1186/1475-2859-10-78. Microb Cell Fact. 2011. PMID: 21981827 Free PMC article.
-
Molecular characterization of pathogenic members of the genus Fonsecaea using multilocus analysis.PLoS One. 2012;7(8):e41512. doi: 10.1371/journal.pone.0041512. Epub 2012 Aug 2. PLoS One. 2012. PMID: 22876287 Free PMC article.
-
Heterologous laccase production and its role in industrial applications.Bioeng Bugs. 2010 Jul-Aug;1(4):252-62. doi: 10.4161/bbug.1.4.11438. Bioeng Bugs. 2010. PMID: 21327057 Free PMC article. Review.
-
Fungal laccases and their applications in bioremediation.Enzyme Res. 2014;2014:163242. doi: 10.1155/2014/163242. Epub 2014 May 15. Enzyme Res. 2014. PMID: 24959348 Free PMC article. Review.
References
-
- Amory, A., et al. September1999. International patent WO9949020A2.
-
- Balance, D. J., F. P. Buxton, and G. Turner. 1983. Transformation of Aspergillus nidulans by the orotidine-5′-phosphate decarboxylase gene. Biochem. Biophys. Res. Commun. 112:284-289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

