The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain
- PMID: 16820517
- PMCID: PMC1489305
- DOI: 10.1128/AEM.03065-05
The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain
Abstract
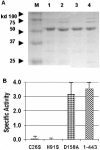
The Streptococcus agalactiae bacteriophage B30 endolysin contains three domains: cysteine, histidine-dependent amidohydrolase/peptidase (CHAP), Acm glycosidase, and the SH3b cell wall binding domain. Truncations and point mutations indicated that the Acm domain requires the SH3b domain for activity, while the CHAP domain is responsible for nearly all the cell lysis activity.
Figures



References
-
- Bateman, A., and N. D. Rawlings. 2003. The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends Biochem. Sci. 28:234-237. - PubMed
-
- Cohen, M. L. 2000. Changing patterns of infectious disease. Nature 406:762-767. - PubMed
-
- De Oliveira, A. P., J. L. Watts, S. A. Salmon, and F. M. Aarestrup. 2000. Antimicrobial susceptibility of Staphylococcus aureus isolated from bovine mastitis in Europe and the United States. J. Dairy Sci. 83:855-862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

