Reconstructing impairment of secretory ameloblast function in porcine teeth by analysis of morphological alterations in dental enamel
- PMID: 16822273
- PMCID: PMC2100299
- DOI: 10.1111/j.1469-7580.2006.00581.x
Reconstructing impairment of secretory ameloblast function in porcine teeth by analysis of morphological alterations in dental enamel
Abstract
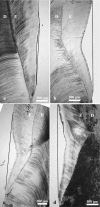
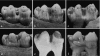
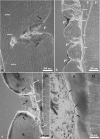
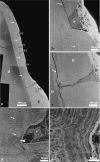
We studied the relationship between the macroscopic appearance of hypoplastic defects in the dental enamel of wild boar and domestic pigs, and microstructural enamel changes, at both the light and the scanning electron microscopic levels. Deviations from normal enamel microstructure were used to reconstruct the functional and related morphological changes of the secretory ameloblasts caused by the action of stress factors during amelogenesis. The deduced reaction pattern of the secretory ameloblasts can be grouped in a sequence of increasingly severe impairments of cell function. The reactions ranged from a slight enhancement of the periodicity of enamel matrix secretion, over a temporary reduction in the amount of secreted enamel matrix, with reduction of the distal portion of the Tomes' process, to either a temporary or a definite cessation of matrix formation. The results demonstrate that analysis of structural changes in dental enamel allows a detailed reconstruction of the reaction of secretory ameloblasts to stress events, enabling an assessment of duration and intensity of these events. Analysing the deviations from normal enamel microstructure provides a deeper insight into the cellular changes underlying the formation of hypoplastic enamel defects than can be achieved by mere inspection of tooth surface characteristics alone.
Figures




Similar articles
-
Disturbances of the secretory stage of amelogenesis in fluorosed deer teeth: a scanning electron-microscopic study.Cell Tissue Res. 1997 Jul;289(1):125-35. doi: 10.1007/s004410050858. Cell Tissue Res. 1997. PMID: 9182607
-
Disturbed enamel formation in wild boars (Sus scrofa L.) from fluoride polluted areas in Central Europe.Anat Rec. 2000 May 1;259(1):12-24. doi: 10.1002/(SICI)1097-0185(20000501)259:1<12::AID-AR2>3.0.CO;2-6. Anat Rec. 2000. PMID: 10760739
-
Insights from the inside: histological analysis of abnormal enamel microstructure associated with hypoplastic enamel defects in human teeth.Am J Phys Anthropol. 2008 Aug;136(4):400-14. doi: 10.1002/ajpa.20822. Am J Phys Anthropol. 2008. PMID: 18350581
-
Cellular and chemical events during enamel maturation.Crit Rev Oral Biol Med. 1998;9(2):128-61. doi: 10.1177/10454411980090020101. Crit Rev Oral Biol Med. 1998. PMID: 9603233 Review.
-
DENTAL ENAMEL FORMATION AND IMPLICATIONS FOR ORAL HEALTH AND DISEASE.Physiol Rev. 2017 Jul 1;97(3):939-993. doi: 10.1152/physrev.00030.2016. Physiol Rev. 2017. PMID: 28468833 Free PMC article. Review.
Cited by
-
Wild boar versus domestic pig-Deciphering of crown growth in porcine second molars.J Anat. 2023 Jun;242(6):1078-1095. doi: 10.1111/joa.13838. Epub 2023 Feb 11. J Anat. 2023. PMID: 36774334 Free PMC article.
-
Rethinking the evidence for early horse domestication at Botai.Sci Rep. 2021 Apr 2;11(1):7440. doi: 10.1038/s41598-021-86832-9. Sci Rep. 2021. PMID: 33811228 Free PMC article.
-
Lines of evidence-incremental markings in molar enamel of Soay sheep as revealed by a fluorochrome labeling and backscattered electron imaging study.PLoS One. 2013 Sep 6;8(9):e74597. doi: 10.1371/journal.pone.0074597. eCollection 2013. PLoS One. 2013. PMID: 24040293 Free PMC article.
-
Early life of Neanderthals.Proc Natl Acad Sci U S A. 2020 Nov 17;117(46):28719-28726. doi: 10.1073/pnas.2011765117. Epub 2020 Nov 2. Proc Natl Acad Sci U S A. 2020. PMID: 33139541 Free PMC article.
-
3D enamel profilometry reveals faster growth but similar stress severity in Neanderthal versus Homo sapiens teeth.Sci Rep. 2021 Jan 12;11(1):522. doi: 10.1038/s41598-020-80148-w. Sci Rep. 2021. PMID: 33436796 Free PMC article.
References
-
- Berten J. Hypoplasien des Schmelzes (Congenitale Schmelzdefecte; Erosionen) Dt Mschr Zahnheilk. 1895;13:425–439. 483–498, 533–548, 587–600.
-
- Boyde A. The surface of the enamel in human hypoplastic teeth. Arch Oral Biol. 1970;15:897–898. - PubMed
-
- Boyde A. Enamel. In: Oksche A, Vollrath L, editors. Handbook of Microscopic Anatomy V/6 Teeth. Heidelberg: Springer; 1989. pp. 309–473.
-
- Boyde A. Microstructure of enamel. In: Chadwick D, Cardew G, editors. Dental Enamel. Ciba Foundation Symposium 205. Chichester: Wiley; 1997. pp. 18–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

