Adaphostin has significant and selective activity against chronic and acute myeloid leukemia cells
- PMID: 16822295
- PMCID: PMC11159172
- DOI: 10.1111/j.1349-7006.2006.00269.x
Adaphostin has significant and selective activity against chronic and acute myeloid leukemia cells
Abstract
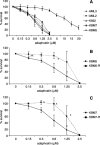
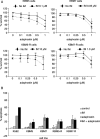
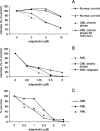
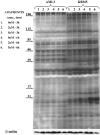
Adaphostin is a tyrphostin that was designed to inhibit Bcr/Abl tyrosine kinase by altering the binding site of peptide substrates rather than that of adenosine triphosphate, a known mechanism of imatinib mesylate (IM). However, it has been shown that adaphostin-mediated cytotoxicity is dependent on oxidant production and does not require Bcr/Abl. We have tested adaphostin against both Philadelphia chromosome (Ph)-positive (K562, KBM5, KBM5-R [IM resistant KBM5], KBM7, and KBM7-R [IM-resistant KBM7]) and Ph-negative (OCI/AML2 and OCI/AML3) cells, and against cells from patients with chronic myeloid leukemia (CML) and acute myeloid leukemia (AML). Adaphostin significantly inhibited growth of all cell lines (50% inhibition of cell proliferation [IC50] 0.5-1 microM) except K562 (IC50 13 microM). Ph-positive IM-resistant cell lines showed significant cross resistance to adaphostin. Simultaneous or sequential treatment with adaphostin and IM did not exert a synergistic effect in any KBM line. Adaphostin induced superoxide and apoptosis in a dose-dependent and time-dependent fashion in both Ph-positive and Ph-negative cells. Adaphostin selectively inhibited colony growth of cells from CML (IM-sensitive and IM-resistant) and AML patients. Analysis of tyrosine phosphorylated proteins after treatment with adaphostin revealed alternate effects in different cells consistent with the modulation of multiple targets. In conclusion, adaphostin showed significant and selective activity against CML and AML cells and its development for clinical testing is warranted.
Figures




Similar articles
-
Effects of the Bcr/abl kinase inhibitors STI571 and adaphostin (NSC 680410) on chronic myelogenous leukemia cells in vitro.Blood. 2002 Jan 15;99(2):664-71. doi: 10.1182/blood.v99.2.664. Blood. 2002. PMID: 11781252
-
Adaphostin-induced oxidative stress overcomes BCR/ABL mutation-dependent and -independent imatinib resistance.Blood. 2006 Mar 15;107(6):2501-6. doi: 10.1182/blood-2005-07-2966. Epub 2005 Nov 15. Blood. 2006. PMID: 16291594 Free PMC article.
-
AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukemia.Clin Cancer Res. 2005 Jul 1;11(13):4941-7. doi: 10.1158/1078-0432.CCR-04-2601. Clin Cancer Res. 2005. PMID: 16000593
-
Characterization of cancer stem cells in chronic myeloid leukaemia.Biochem Soc Trans. 2007 Nov;35(Pt 5):1347-51. doi: 10.1042/BST0351347. Biochem Soc Trans. 2007. PMID: 17956348 Review.
-
Targeted chronic myeloid leukemia therapy: seeking a cure.J Manag Care Pharm. 2007 Oct;13(8 Suppl A):8-12. doi: 10.18553/jmcp.2007.13.s8-a.8. J Manag Care Pharm. 2007. PMID: 17970609 Free PMC article. Review.
Cited by
-
Hematopoietic Stem Cells: Normal Versus Malignant.Antioxid Redox Signal. 2018 Dec 1;29(16):1612-1632. doi: 10.1089/ars.2017.7326. Epub 2017 Dec 20. Antioxid Redox Signal. 2018. PMID: 29084438 Free PMC article. Review.
-
The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives.Chem Rev. 2013 May 8;113(5):3516-604. doi: 10.1021/cr100264t. Epub 2013 Feb 25. Chem Rev. 2013. PMID: 23432396 Free PMC article. Review. No abstract available.
-
Reactive Oxygen Species in Acute Lymphoblastic Leukaemia: Reducing Radicals to Refine Responses.Antioxidants (Basel). 2021 Oct 14;10(10):1616. doi: 10.3390/antiox10101616. Antioxidants (Basel). 2021. PMID: 34679751 Free PMC article. Review.
-
Evaluation of Different Concentrations of Imatinib on the Viability of Leishmania major: An In Vitro Study.Adv Biomed Res. 2019 Oct 31;8:61. doi: 10.4103/abr.abr_58_19. eCollection 2019. Adv Biomed Res. 2019. PMID: 31737578 Free PMC article.
-
Tyrosine Kinase Inhibitor Profiling Using Multiple Forskolin-Responsive Reporter Cells.Int J Mol Sci. 2023 Sep 8;24(18):13863. doi: 10.3390/ijms241813863. Int J Mol Sci. 2023. PMID: 37762164 Free PMC article.
References
-
- Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. New Engl J Med 1999; 341: 164–72. - PubMed
-
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 1990; 247: 824–30. - PubMed
-
- Druker BJ, Tamura S, Buchdunger E et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr‐Abl positive cells. Nat Med 1996; 2: 561–6. - PubMed
-
- Beran M, Cao X, Estrov Z et al. Selective inhibition of cell proliferation and BCR‐ABL phosphorylation in acute lymphoblastic leukemia cells expressing Mr 190 000 BCR‐ABL protein by a tyrosine kinase inhibitor (CGP‐57148). Clin Cancer Res 1998; 4: 1661–72. - PubMed
-
- Deininger MW, Goldman JM, Lydon N, Melo JV. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR‐ABL‐positive cells. Blood 1997; 90: 3691–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

