Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors
- PMID: 16822975
- PMCID: PMC6673951
- DOI: 10.1523/JNEUROSCI.1258-06.2006
Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors
Abstract
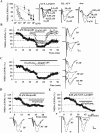
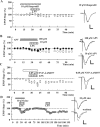
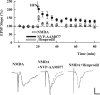
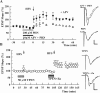
Zinc has complex effects on NMDA receptors (NMDARs) and may be an endogenous modulator of synaptic plasticity. In the CA1 region of rat hippocampal slices, we observed that low micromolar concentrations of zinc depress NMDAR synaptic responses by 40-50% and inhibit long-term depression (LTD) but not long-term potentiation (LTP). A combination of zinc plus ifenprodil, an inhibitor of NR1/NR2B receptors, produced no greater inhibition of synaptic NMDARs than either agent alone, suggesting overlapping effects on NMDARs. Similar to low micromolar zinc, ifenprodil inhibited LTD but not LTP. In contrast, low concentrations of 2-amino-5-phosphonovalerate (APV) did not block either LTP or LTD despite producing >50% inhibition of synaptic NMDARs. NVP-AAM077 ([(R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydro-quinoxalin-5-yl)-methyl]phosphonic acid), an antagonist with relative NR1/NR2A selectivity at low concentrations, also inhibited synaptic NMDARs by approximately 50% at 0.05 mum but failed to completely block either LTP or LTD. These results suggest that LTD induction depends on specific NMDARs with sensitivity to low micromolar zinc and ifenprodil, but LTP is less dependent on specific NMDAR subtypes. Because high-affinity sites of NR2A are likely occupied by ambient zinc, we also examined effects of extracellular zinc chelators. Zinc chelation blocked LTP but had no effect on LTD. This LTP inhibition was overcome by APV and NVP-AAM077 but not ifenprodil, suggesting that zinc chelation unmasks tonic NR1/NR2A activation that negatively modulates LTP.
Figures


Similar articles
-
Sensitivity of N-methyl-D-aspartate receptor-mediated excitatory postsynaptic potentials and synaptic plasticity to TCN 201 and TCN 213 in rat hippocampal slices.J Pharmacol Exp Ther. 2015 Feb;352(2):267-73. doi: 10.1124/jpet.114.220582. Epub 2014 Nov 20. J Pharmacol Exp Ther. 2015. PMID: 25413830 Free PMC article.
-
Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression.J Neurosci. 2006 Feb 8;26(6):1721-9. doi: 10.1523/JNEUROSCI.3990-05.2006. J Neurosci. 2006. PMID: 16467520 Free PMC article.
-
Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus.Neuropharmacology. 2007 Jan;52(1):60-70. doi: 10.1016/j.neuropharm.2006.07.013. Epub 2006 Aug 10. Neuropharmacology. 2007. PMID: 16904707
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
-
NMDA receptors and synaptic plasticity in the anterior cingulate cortex.Neuropharmacology. 2021 Oct 1;197:108749. doi: 10.1016/j.neuropharm.2021.108749. Epub 2021 Aug 5. Neuropharmacology. 2021. PMID: 34364898 Review.
Cited by
-
The Role of Zinc and NMDA Receptors in Autism Spectrum Disorders.Pharmaceuticals (Basel). 2022 Dec 20;16(1):1. doi: 10.3390/ph16010001. Pharmaceuticals (Basel). 2022. PMID: 36678498 Free PMC article. Review.
-
GluN2B subunit-containing NMDA receptor antagonists prevent Abeta-mediated synaptic plasticity disruption in vivo.Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20504-9. doi: 10.1073/pnas.0908083106. Epub 2009 Nov 16. Proc Natl Acad Sci U S A. 2009. PMID: 19918059 Free PMC article.
-
Acrylamide inhibits long-term potentiation and learning involving microglia and pro-inflammatory signaling.Sci Rep. 2022 Jul 20;12(1):12429. doi: 10.1038/s41598-022-16762-7. Sci Rep. 2022. PMID: 35858988 Free PMC article.
-
Zinc enhances hippocampal long-term potentiation at CA1 synapses through NR2B containing NMDA receptors.PLoS One. 2018 Nov 28;13(11):e0205907. doi: 10.1371/journal.pone.0205907. eCollection 2018. PLoS One. 2018. PMID: 30485271 Free PMC article.
-
Dietary Zinc Supplementation Prevents Autism Related Behaviors and Striatal Synaptic Dysfunction in Shank3 Exon 13-16 Mutant Mice.Front Cell Neurosci. 2018 Oct 22;12:374. doi: 10.3389/fncel.2018.00374. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30405356 Free PMC article.
References
-
- Assaf SY, Chung SH (1984). Release of endogenous Zn2+ from brain tissue during activity. Nature 308:734–736. - PubMed
-
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M (2002). 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett 12:1099–1102. - PubMed
-
- Chen N, Moshaver A, Raymond LA (1997). Differential sensitivity of recombinant N-methyl-d-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol 51:1015–1023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
