Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF-deficient hippocampal neurons
- PMID: 16822976
- PMCID: PMC6673958
- DOI: 10.1523/JNEUROSCI.5474-05.2006
Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF-deficient hippocampal neurons
Abstract
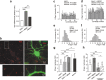
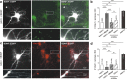
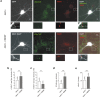
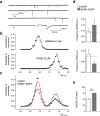
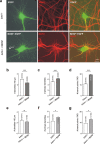
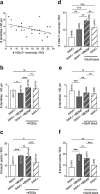
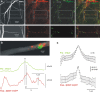
Cultured neurons from bdnf-/- mice display reduced densities of synaptic terminals, although in vivo these deficits are small or absent. Here we aimed at clarifying the local responses to postsynaptic brain-derived neurotrophic factor (BDNF). To this end, solitary enhanced green fluorescent protein (EGFP)-labeled hippocampal neurons from bdnf-/- mice were compared with bdnf-/- neurons after transfection with BDNF, bdnf-/- neurons after transient exposure to exogenous BDNF, and bdnf+/+ neurons in wild-type cultures. Synapse development was evaluated on the basis of presynaptic immunofluorescence and whole-cell patch-clamp recording of miniature postsynaptic currents. It was found that neurons expressing BDNF::EGFP for at least 16 h attracted a larger number of synaptic terminals than BDNF-deficient control neurons. Transfected BDNF formed clusters in the vicinity of glutamatergic terminals and produced a stronger upregulation of synaptic terminal numbers than high levels of ambient BDNF. Glutamatergic and GABAergic synapses reacted differently to postsynaptic BDNF: glutamatergic input increased, whereas GABAergic input decreased. BDNF::EGFP-expressing neurons also differed from BDNF-deficient neurons in their dendrite morphology: they exhibited weaker dendrite elongation and stronger dendrite initiation. The upregulation of glutamatergic synaptic input and the BDNF-induced downregulation of GABAergic synaptic terminal numbers by postsynaptic BDNF depended on tyrosine receptor kinase B activity, as deduced from the blocking effects of K252a. The suppression of dendrite elongation was also prevented by block of tyrosine receptor kinase B but required, in addition, glutamate receptor activity. Dendritic length decreased with the number of glutamatergic contacts. These results illuminate the role of BDNF as a retrograde synaptic regulator of synapse development and the dependence of dendrite elongation on glutamatergic input.
Figures
Similar articles
-
Glutamatergic Innervation onto Striatal Neurons Potentiates GABAergic Synaptic Output.J Neurosci. 2019 Jun 5;39(23):4448-4460. doi: 10.1523/JNEUROSCI.2630-18.2019. Epub 2019 Apr 1. J Neurosci. 2019. PMID: 30936241 Free PMC article.
-
Impaired transmission at corticothalamic excitatory inputs and intrathalamic GABAergic synapses in the ventrobasal thalamus of heterozygous BDNF knockout mice.Neuroscience. 2012 Oct 11;222:215-27. doi: 10.1016/j.neuroscience.2012.07.005. Epub 2012 Jul 13. Neuroscience. 2012. PMID: 22796079
-
Synaptic reliability correlates with reduced susceptibility to synaptic potentiation by brain-derived neurotrophic factor.Learn Mem. 1999 May-Jun;6(3):232-42. Learn Mem. 1999. PMID: 10492005 Free PMC article.
-
Role of the brain-derived neurotrophic factor at glutamatergic synapses.Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S310-24. doi: 10.1038/sj.bjp.0707509. Epub 2007 Dec 3. Br J Pharmacol. 2008. PMID: 18059328 Free PMC article. Review.
-
BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses.Exp Brain Res. 2009 Dec;199(3-4):203-34. doi: 10.1007/s00221-009-1994-z. Epub 2009 Sep 24. Exp Brain Res. 2009. PMID: 19777221 Review.
Cited by
-
Serum brain-derived neurotrophic factor levels in different neurological diseases.Biomed Res Int. 2013;2013:901082. doi: 10.1155/2013/901082. Epub 2013 Aug 19. Biomed Res Int. 2013. PMID: 24024214 Free PMC article.
-
The CaV1.2 G406R mutation decreases synaptic inhibition and alters L-type Ca2+ channel-dependent LTP at hippocampal synapses in a mouse model of Timothy Syndrome.Neuropharmacology. 2022 Dec 1;220:109271. doi: 10.1016/j.neuropharm.2022.109271. Epub 2022 Sep 24. Neuropharmacology. 2022. PMID: 36162529 Free PMC article.
-
Computer Simulations Support a Morphological Contribution to BDNF Enhancement of Action Potential Generation.Front Cell Neurosci. 2016 Sep 14;10:209. doi: 10.3389/fncel.2016.00209. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27683544 Free PMC article.
-
Effects of exogenous agents on brain development: stress, abuse and therapeutic compounds.CNS Neurosci Ther. 2011 Oct;17(5):470-89. doi: 10.1111/j.1755-5949.2010.00171.x. Epub 2010 Jun 14. CNS Neurosci Ther. 2011. PMID: 20553311 Free PMC article. Review.
-
Gene expression patterns in the hippocampus during the development and aging of Glud1 (Glutamate Dehydrogenase 1) transgenic and wild type mice.BMC Neurosci. 2014 Mar 4;15:37. doi: 10.1186/1471-2202-15-37. BMC Neurosci. 2014. PMID: 24593767 Free PMC article.
References
-
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E (2003). BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K(+)/Cl(−) cotransporter KCC2. Development 130:1267–1280. - PubMed
-
- Bi G, Poo MM (2001). Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci 24:139–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
