Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression
- PMID: 16822986
- PMCID: PMC6673936
- DOI: 10.1523/JNEUROSCI.1072-06.2006
Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression
Abstract
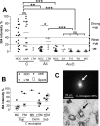
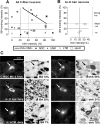
Binding to isolectin-B4 (IB4) and expression of tyrosine kinase A (trkA) (the high-affinity NGF receptor) have been used to define two different subgroups of nociceptive small dorsal root ganglion (DRG) neurons. We previously showed that only nociceptors have high trkA levels. However, information about sensory and electrophysiological properties in vivo of single identified IB4-binding neurons, and about their trkA expression levels, is lacking. IB4-positive (IB4+) and small dark neurons had similar size distributions. We examined IB4-binding levels in >120 dye-injected DRG neurons with sensory and electrophysiological properties recorded in vivo. Relative immunointensities for trkA and two TTX-resistant sodium channels (Nav1.8 and Nav1.9) were also measured in these neurons. IB4+ neurons were classified as strongly or weakly IB4+. All strongly IB4+ neurons were C-nociceptor type (C-fiber nociceptive or unresponsive). Of 32 C-nociceptor-type neurons examined, approximately 50% were strongly IB4+, approximately 20% were weakly IB4+ and approximately 30% were IB4-. Adelta low-threshold mechanoreceptive (LTM) neurons were weakly IB4+ or IB4-. All 33 A-fiber nociceptors and all 44 Aalpha/beta-LTM neurons examined were IB4-. IB4+ compared with IB4- C-nociceptor-type neurons had longer somatic action potential durations and rise times, slower conduction velocities, more negative membrane potentials, and greater immunointensities for Nav1.9 but not Nav1.8. Immunointensities of IB4 binding in C-neurons were positively correlated with those of Nav1.9 but not Nav1.8. Of 23 C-neurons tested for both trkA and IB4, approximately 35% were trkA+/IB4+ but with negatively correlated immunointensities; 26% were IB4+/trkA-, and 35% were IB4-/trkA+. We conclude that strongly IB4+ DRG neurons are exclusively C-nociceptor type and that high Nav1.9 expression may contribute to their distinct membrane properties.
Figures




Similar articles
-
trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors.J Neurosci. 2005 May 11;25(19):4868-78. doi: 10.1523/JNEUROSCI.0249-05.2005. J Neurosci. 2005. PMID: 15888662 Free PMC article.
-
The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons.J Physiol. 2003 Aug 1;550(Pt 3):739-52. doi: 10.1113/jphysiol.2003.042127. Epub 2003 Jun 6. J Physiol. 2003. PMID: 12794175 Free PMC article.
-
Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons.Brain Res Mol Brain Res. 1999 Apr 20;67(2):267-82. doi: 10.1016/s0169-328x(99)00070-4. Brain Res Mol Brain Res. 1999. PMID: 10216225
-
The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons.J Neurosci. 2002 Sep 1;22(17):7425-33. doi: 10.1523/JNEUROSCI.22-17-07425.2002. J Neurosci. 2002. PMID: 12196564 Free PMC article.
-
Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres.Exp Physiol. 2002 Mar;87(2):239-44. Exp Physiol. 2002. PMID: 11856969 Review.
Cited by
-
Despite differences in cytosolic calcium regulation, lidocaine toxicity is similar in adult and neonatal rat dorsal root ganglia in vitro.Anesthesiology. 2014 Jan;120(1):50-61. doi: 10.1097/ALN.0b013e3182a2a561. Anesthesiology. 2014. PMID: 23851347 Free PMC article.
-
Antinociceptive modulation by the adhesion GPCR CIRL promotes mechanosensory signal discrimination.Elife. 2020 Sep 30;9:e56738. doi: 10.7554/eLife.56738. Elife. 2020. PMID: 32996461 Free PMC article.
-
Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse.J Pain. 2007 Mar;8(3):263-72. doi: 10.1016/j.jpain.2006.09.005. Epub 2006 Nov 16. J Pain. 2007. PMID: 17113352 Free PMC article.
-
The Importance of Peripheral Nerves in Adipose Tissue for the Regulation of Energy Balance.Biology (Basel). 2019 Feb 12;8(1):10. doi: 10.3390/biology8010010. Biology (Basel). 2019. PMID: 30759876 Free PMC article. Review.
-
Complex alterations in inflammatory pain and analgesic sensitivity in young and ageing female rats: involvement of ASIC3 and Nav1.8 in primary sensory neurons.Inflamm Res. 2024 Apr;73(4):669-691. doi: 10.1007/s00011-024-01862-z. Epub 2024 Mar 14. Inflamm Res. 2024. PMID: 38483556
References
-
- Bogen O, Dreger M, Gillen C, Schroder W, Hucho F (2005). Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. FEBS J 272:1090–1102. - PubMed
-
- Braz JM, Nassar MA, Wood JN, Basbaum AI (2005). Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 47:787–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
