The L1 retrotranspositional stimulation by particulate and soluble cadmium exposure is independent of the generation of DNA breaks
- PMID: 16823085
- PMCID: PMC3807503
- DOI: 10.3390/ijerph2006030015
The L1 retrotranspositional stimulation by particulate and soluble cadmium exposure is independent of the generation of DNA breaks
Abstract
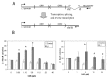
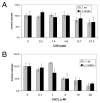
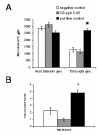
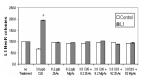
Human exposure to toxic metals is a concern of the highest priority, due to their vast array of biological effects, including carcinogenicity. The particulate (water insoluble) form of several heavy metals presents a higher carcinogenic potential than its soluble counterparts. Our previous work demonstrates that the particulate forms of different heavy metals, such as nickel oxide, cadmium sulfide and mercury sulfide, stimulate human L1 mobile element activity leading to genomic instability. We present data demonstrating that the soluble form of CdCl2 also stimulates L1 retrotransposition in a dose-dependent manner comparable to the insoluble carcinogenic form of this compound. Reproducible results demonstrated a 2 to 3 fold dose-dependent increase in L1 retrotransposition compared to control cells. Heavy metals may cause DNA breaks through the generation of reactive oxygen species. However, evaluation of DNA damage by comet assay revealed no differences between the negative controls and the CdS-treated cells. In addition, active L1 elements express a protein with endonuclease activity that can generate toxicity through the creation of double strand breaks. To determine the contribution of the L1 endonuclease to the toxicity observed in our metal treatment assays, we compared the wildtype L1 vector with an L1 endonuclease-mutant vector. The presence of an active L1 endonuclease did not contribute significantly to the toxicity observed in any of the CdCl2 or CdS doses evaluated. No correlation between the creation of DNA breaks and L1 activity was observed. Alternatively, heavy metals inhibit enzymatic reactions by displacement of cofactors such as Zn and Mg from enzymes. Concomitant treatment with Mg(Ac)2 and Zn(Ac)2 ppb suppresses the stimulatory effect on L1 activity induced by the 3.8 ppb CdS treatment. Overall, these results are consistent with our previous observations, suggesting that the mechanism of L1 stimulation by heavy metals is most likely due to an overall inhibition of DNA repair proteins or other enzymes caused by the displacement of Mg and Zn from cellular proteins.
Figures
References
-
- Meeting of the IARC working group on beryllium, cadmium, mercury and exposures in the glass manufacturing industry. Scand. J. Work Environ. Health. 1993;19(5):360–363. - PubMed
-
- Waalkes M. P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000;79(1–4):241–244. - PubMed
-
- Takenaka S., Oldiges H., Konig H., Hochrainer D., Oberdorster G. Carcinogenicity of cadmium chloride aerosols in W rats. J. Natl. Cancer Inst. 1983;70(2):367–373. - PubMed
-
- Costa M., Sutherland J. E., Peng W., Salnikow K., Broday L., Kluz T. Molecular biology of nickel carcinogenesis. Mol. Cell Biochem. 2001;222(1–2):205–211. - PubMed
-
- Szuster-Ciesielska A., et al. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology. 2000;145(2–3):159–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
