Dose- and time-dependent response of human leukemia (HL-60) cells to arsenic trioxide treatment
- PMID: 16823087
- PMCID: PMC3807505
- DOI: 10.3390/ijerph2006030017
Dose- and time-dependent response of human leukemia (HL-60) cells to arsenic trioxide treatment
Abstract
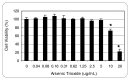
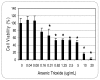
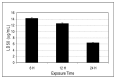
The treatment of acute promyelocytic leukemia (APL) has been based on the administration of all-trans retinoic acid plus anthracycline chemotherapy, which is very effective as first line therapy; however 25 to 30% of patients will relapse with their disease becoming refractory to conventional therapy. Recently, studies have shown arsenic trioxide to be effective in the treatment of acute promyelocytic leukemia. In this study, we used the human leukemia (HL-60) cell line as a model to evaluate the cytoxicity of arsenic trioxide based on the MTT assay. Data obtained from this assay indicated that arsenic trioxide significantly reduced the viability of HL-60 cells, showing LD50 values of 14.26 + 0.5microg/mL, 12.54 + 0.3microg/mL, and 6.4 + 0.6microg/mL upon 6, 12, and 24 hours of exposure, respectively; indicating a dose- and time-dependent response relationship. Findings from the present study indicate that arsenic trioxide is highly cytotoxic to human leukemia (HL-60) cells, supporting its use as an effective therapeutic agent in the management of acute promyelocytic leukemia.
Figures


Similar articles
-
Arsenic trioxide as first-line treatment for acute promyelocytic leukemia.Am J Health Syst Pharm. 2009 Nov 1;66(21):1913-8. doi: 10.2146/ajhp080342. Am J Health Syst Pharm. 2009. PMID: 19850784
-
Basic mechanisms of arsenic trioxide (ATO)-induced apoptosis in human leukemia (HL-60) cells.J Hematol Oncol. 2010 Aug 26;3:28. doi: 10.1186/1756-8722-3-28. J Hematol Oncol. 2010. PMID: 20796291 Free PMC article.
-
Differential expression and response to arsenic stress of MRPs and ASAN1 determine sensitivity of classical multidrug-resistant leukemia cells to arsenic trioxide.Leuk Res. 2016 Nov;50:116-122. doi: 10.1016/j.leukres.2016.10.003. Epub 2016 Oct 3. Leuk Res. 2016. PMID: 27736728
-
Arsenic-induced apoptosis in malignant cells in vitro.Leuk Lymphoma. 2000 Mar;37(1-2):53-63. doi: 10.3109/10428190009057628. Leuk Lymphoma. 2000. PMID: 10721769 Review.
-
Differentiating agents in pediatric malignancies: all-trans-retinoic acid and arsenic in acute promyelocytic leukemia.Curr Oncol Rep. 2000 Nov;2(6):519-23. doi: 10.1007/s11912-000-0105-x. Curr Oncol Rep. 2000. PMID: 11122887 Review.
Cited by
-
Arsenic trioxide induces apoptosis of p53 null osteosarcoma MG63 cells through the inhibition of catalase.Med Oncol. 2012 Jun;29(2):1328-34. doi: 10.1007/s12032-011-9848-5. Epub 2011 Feb 10. Med Oncol. 2012. PMID: 21308489
-
ASCORBIC ACID - MODULATION OF ARSENIC TRIOXIDE TOXICITY: IMPLICATION FOR THE CLINICAL TREATMENT OF ACUTE PROMYELOCYTIC LEUKEMIA.Met Ions Biol Med. 2008;10:413-418. Met Ions Biol Med. 2008. PMID: 26549974 Free PMC article.
-
Antileukemic Potential of Momordica charantia Seed Extracts on Human Myeloid Leukemic HL60 Cells.Evid Based Complement Alternat Med. 2012;2012:732404. doi: 10.1155/2012/732404. Epub 2012 May 13. Evid Based Complement Alternat Med. 2012. PMID: 22654956 Free PMC article.
-
N-acetyl-l-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells.Int J Environ Res Public Health. 2007 Jun;4(2):132-7. doi: 10.3390/ijerph2007040007. Int J Environ Res Public Health. 2007. PMID: 17617676 Free PMC article.
-
Health-Promoting Properties of Borage Seed Oil Fractionated by Supercritical Carbon Dioxide Extraction.Foods. 2021 Oct 15;10(10):2471. doi: 10.3390/foods10102471. Foods. 2021. PMID: 34681520 Free PMC article.
References
-
- Antman K H. The history of arsenic trioxide in cancer therapy. The Oncologist. 2001;6(2):1–2. - PubMed
-
- Waxman S., Anderson K C. History of the development of arsenic derivatives in cancer therapy. The Oncologist. 2001;6(2):3–10. - PubMed
-
- Soignet S. L., Maslak P., Wang Z-G., Jhanwar S., Calleja E., Dardashti L. J., Corso D., DeBlasio A., Gabrilove J., Scheinberg D. A., Pandolfi P. P., Warrell R. P. Complete Remission after Treatment of Acute Promyelocytic Leukemia with Arsenic Trioxide. N Engl J Med. 1998;339:1341–1348. - PubMed
-
- Soignet S. L., Frankel S. R., Douer D., Tallman M. S., Kantarjian H., Calleja E., Stone R. M., Kalaycio M., Scheinberg D. A., Steinherz P., Sievers E. L., Coutré S., Dahlberg S., Ellison R., Warrell R. P., Jr United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J. Clin. Oncol. 2001;19:3852–3860. - PubMed
-
- Warrell R. P., Jr. Pathogenesis and management of acute promyelocytic leukemia. Annu Rev Med. 1996;47:555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
