Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines
- PMID: 16823487
- PMCID: PMC1483161
- DOI: 10.1172/JCI27019
Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines
Abstract
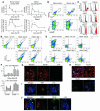
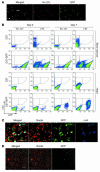
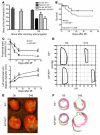
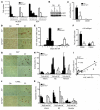
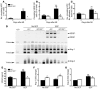
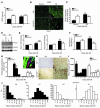
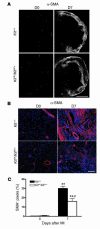
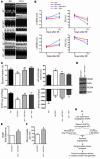
Clinical trials of bone marrow stem/progenitor cell therapy after myocardial infarction (MI) have shown promising results, but the mechanism of benefit is unclear. We examined the nature of endogenous myocardial repair that is dependent on the function of the c-kit receptor, which is expressed on bone marrow stem/progenitor cells and on recently identified cardiac stem cells. MI increased the number of c-kit+ cells in the heart. These cells were traced back to a bone marrow origin, using genetic tagging in bone marrow chimeric mice. The recruited c-kit+ cells established a proangiogenic milieu in the infarct border zone by increasing VEGF and by reversing the cardiac ratio of angiopoietin-1 to angiopoietin-2. These oscillations potentiated endothelial mitogenesis and were associated with the establishment of an extensive myofibroblast-rich repair tissue. Mutations in the c-kit receptor interfered with the mobilization of the cells to the heart, prevented angiogenesis, diminished myofibroblast-rich repair tissue formation, and led to precipitous cardiac failure and death. Replacement of the mutant bone marrow with wild-type cells rescued the cardiomyopathic phenotype. We conclude that, consistent with their documented role in tumorigenesis, bone marrow c-kit+ cells act as key regulators of the angiogenic switch in infarcted myocardium, thereby driving efficient cardiac repair.
Figures
Comment in
-
Lost and found: cardiac stem cell therapy revisited.J Clin Invest. 2006 Jul;116(7):1838-40. doi: 10.1172/JCI29050. J Clin Invest. 2006. PMID: 16823485 Free PMC article.
Similar articles
-
Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction.Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2304-9. doi: 10.1073/pnas.0510997103. Epub 2006 Feb 7. Proc Natl Acad Sci U S A. 2006. PMID: 16467148 Free PMC article.
-
c-kit dysfunction impairs myocardial healing after infarction.Circulation. 2007 Sep 11;116(11 Suppl):I77-82. doi: 10.1161/CIRCULATIONAHA.107.708107. Circulation. 2007. PMID: 17846329
-
Apelin enhances cardiac neovascularization after myocardial infarction by recruiting aplnr+ circulating cells.Circ Res. 2012 Aug 17;111(5):585-98. doi: 10.1161/CIRCRESAHA.111.262097. Epub 2012 Jul 2. Circ Res. 2012. PMID: 22753078
-
Restoration of cardiac function with progenitor cells.Novartis Found Symp. 2006;274:214-23; discussion 223-7, 272-6. Novartis Found Symp. 2006. PMID: 17019814 Review.
-
Endothelial progenitor cells functional characterization.Trends Cardiovasc Med. 2004 Nov;14(8):318-22. doi: 10.1016/j.tcm.2004.10.001. Trends Cardiovasc Med. 2004. PMID: 15596109 Review.
Cited by
-
Electroacupuncture improves behavioral recovery and increases SCF/c-kit expression in a rat model of focal cerebral ischemia/reperfusion.Neurol Sci. 2013 Apr;34(4):487-95. doi: 10.1007/s10072-012-1081-2. Epub 2012 Apr 13. Neurol Sci. 2013. PMID: 22526758
-
Regenerating new heart with stem cells.J Clin Invest. 2013 Jan;123(1):62-70. doi: 10.1172/JCI63068. Epub 2013 Jan 2. J Clin Invest. 2013. Retraction in: J Clin Invest. 2018 Dec 3;128(12):5676. doi: 10.1172/JCI126075. PMID: 23281411 Free PMC article. Retracted. Review.
-
Mechanisms of the protective effects of BMSCs promoted by TMDR with heparinized bFGF-incorporated stent in pig model of acute myocardial ischemia.J Cell Mol Med. 2011 May;15(5):1075-86. doi: 10.1111/j.1582-4934.2010.01070.x. Epub 2010 Apr 7. J Cell Mol Med. 2011. PMID: 20406325 Free PMC article.
-
Stem cells and cardiac repair: a critical analysis.J Cardiovasc Transl Res. 2008 Mar;1(1):41-54. doi: 10.1007/s12265-007-9008-7. Epub 2008 Jan 31. J Cardiovasc Transl Res. 2008. PMID: 20559957 Review.
-
Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction.Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2604-9. doi: 10.1073/pnas.0914138107. Epub 2010 Jan 21. Proc Natl Acad Sci U S A. 2010. PMID: 20133784 Free PMC article.
References
-
- Orlic D., et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. - PubMed
-
- Fazel S., et al. Current status of cellular therapy for ischemic heart disease. Ann. Thorac. Surg. 2005;79:S2238–S2247. - PubMed
-
- Balsam L.B., et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. - PubMed
-
- Murry C.E., et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

