Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity
- PMID: 16824126
- PMCID: PMC7165519
- DOI: 10.1111/j.0105-2896.2006.00394.x
Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity
Abstract
The main functions of memory T cells are to provide protection upon re-exposure to a pathogen and to prevent the re-emergence of low-grade persistent pathogens. Memory T cells achieve these functions through their high frequency and elevated activation state, which lead to rapid responses upon antigenic challenge. The significance and characteristics of memory CD8+ T cells in viral infections have been studied extensively. In many of these studies of T-cell memory, experimental viral immunologists go to great lengths to assure that their animal colonies are free of endogenous pathogens in order to design reproducible experiments. These experimental results are then thought to provide the basis for our understanding of human immune responses to viruses. Although these findings can be enlightening, humans are not immunologically naïve, and they often have memory T-cell populations that can cross-react with and respond to a new infectious agent or cross-react with allo-antigens and influence the success of tissue transplantation. These cross-reactive T cells can become activated and modulate the immune response and outcome of subsequent heterologous infections, a phenomenon we have termed heterologous immunity. These large memory populations are also accommodated into a finite immune system, requiring that the host makes room for each new population of memory cell. It appears that memory cells are part of a continually evolving interactive network, where with each new infection there is an alteration in the frequencies, distributions, and activities of memory cells generated in response to previous infections and allo-antigens.
Figures
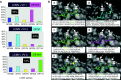
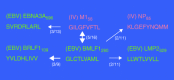

References
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science 1996;272: 54–60. - PubMed
-
- Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol 1998;16: 201–223. - PubMed
-
- Zinkernagel RM. On differences between immunity and immunological memory. Curr Opin Immunol 2002;14: 523–536. - PubMed
-
- Pewe LL, Netland JM, Heard SB, Perlman S. Very diverse CD8 T cell clonotypic responses after virus infections. J Immunol 2004;172: 3151–3156. - PubMed
-
- Naumov YN, Naumova EN, Hogan KT, Selin LK, Gorski J. A fractal clonotype distribution in the CD8+ memory T cell repertoire could optimize potential for immune responses. J Immunol 2003;170: 3994–4001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

