Understanding and making use of human memory B cells
- PMID: 16824137
- PMCID: PMC7165660
- DOI: 10.1111/j.0105-2896.2006.00403.x
Understanding and making use of human memory B cells
Abstract
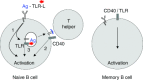
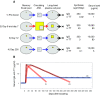
The work of our laboratory has focused on the study of human memory B cells. Using an in vitro approach we dissected the triggering requirements of B cells and unveiled a distinct role for TLRs in the activation of naive versus memory B cells. Using an ex vivo approach we analyzed the dynamics of memory B cells and ASCs and the kinetics of serum antibodies during secondary immune responses and in steady state conditions and used these quantitative data to build up a model of serological memory. According to this model memory B cells behave as ;stem cells' capable of generating plasma cells and antibodies in an antigen-dependent as well as in an antigen-independent fashion. Finally we developed an efficient method to interrogate human memory B cells and to isolate human monoclonal antibodies. This method can be exploited for the production of neutralizing antibodies for serotherapy and for "analytic vaccinology".
Figures

References
-
- Mills DM, Cambier JC. B lymphocyte activation during cognate interactions with CD4+ T lymphocytes: molecular dynamics and immunologic consequences. Semin Immunol 2003;15: 325–329. - PubMed
-
- Parker DC. T cell‐dependent B cell activation. Annu Rev Immunol 1993;11: 331–360. - PubMed
-
- Banchereau J, et al. The CD40 antigen and its ligand. Annu Rev Immunol 1994;12: 881–922. - PubMed
-
- Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol 2005;23: 367–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

